Abstract
Purpose: This study investigates the influence of blood perfusion variability within a tumour and the surrounding healthy tissue during nanoparticle-assisted thermal therapy. It seeks to define ideal therapeutic parameters for a wide range of perfusion rates to attain the desired thermal damage. Material and methods: Pennes’ bioheat model and the Arrhenius model are used to evaluate the thermal damage for a two-dimensional tumour surrounded by healthy tissue. A wide range of tumour perfusion rates were modelled, ranging from moderate to high perfusion in both a homogenously and a heterogeneously perfused tumour. Results: For low perfusion rates, a temporal variation in blood perfusion does not critically influence the thermal damage. For moderately and highly perfused tumours, temporal variation in blood perfusion extends the thermal damage zone by 25–52% compared to a constant perfusion rate. For the tumour size and perfusion conditions under consideration, the ideal therapeutic parameters were found to be irradiation intensity of 1 W/cm2, and irradiation duration of 105–150 s, for a nanoparticle volume fraction of 0.001%. Conclusions: It is concluded for low perfusion rates that due to shorter therapeutic duration, nanoparticle-assisted thermal therapy is relatively insensitive to changes in the perfusion rate during the therapy. For moderately and highly perfused tumours, a constant perfusion under-predicts the real thermal damage zone. This study concludes that for moderately and highly perfused tumours the spatial as well as temporal blood perfusion dynamics should be carefully accounted for to get a realistic estimate of thermal damage zone.
Introduction
Nanoparticle-assisted thermal therapy is evolving as a promising future treatment for cancer [Citation1–5]. This therapy employs nanoparticles as heat sources within a tumour to selectively kill the tumour cells through thermal ablation [Citation1,Citation6]. This therapy utilises the wavelength band known as the ‘therapeutic window’ (600 nm–1300 nm) [Citation7]. Within this wavelength band, the tissue absorbs radiation minimally while the nanoparticles strongly absorb it, thus considerably heating the tumour from within to cause thermal damage. Another important form of thermal therapy (currently undergoing clinical testing) utilises ferromagnetic nanoparticles to generate thermal energy for thermal ablation [Citation8–10].
Blood flow within the tissue acts as a negative feedback mechanism to transfer heat away from the tissue to thermo-regulate healthy tissue [Citation11]. Thus, blood flow significantly influences thermal ablation temperatures and the extent of thermal damage achieved within and around a tumour. Tumours are characterised by heterogeneous vasculature [Citation12–14] and large variation of blood perfusion, even within tumours of the same type [Citation15–18]. Additionally, during heating, temporal variation in the blood perfusion rate occurs as the body’s response to blood vessels experiencing thermal damage [Citation19].
This issue of blood perfusion has been addressed widely for radiofrequency (RF) ablation and hyperthermia [Citation15,Citation20–22]. However, for nanoparticle-assisted thermal therapy, there exists little specific research that quantifies the role of blood perfusion. Given the considerably different heating regimes of this alternative treatment, a fundamental understanding of the influence of blood perfusion under typical nanoparticle-assisted therapy conditions is needed. This study represents the first effort in this direction and serves to quantify the extent of this issue by examining a wide range of parameters. Additionally, this research adds to further understanding in the area of gold nanorod-assisted thermal therapy [Citation1–6,Citation23]. As such, this study highlights the role of the spatio-temporal variability of blood perfusion within the tumour as well as the surrounding healthy tissue during these types of treatments. The temporal variation of blood perfusion is incorporated based on vascular stasis. The spatial variation of the perfusion rate is also incorporated by comparing a homogenously perfused tumour with a heterogeneously perfused tumour under similar irradiation conditions. The spatio-temporal thermal ablation temperatures and thermal damage are evaluated based on Pennes’ bioheat equation and the Arrhenius model. For each configuration, ideal therapeutic parameters (i.e. irradiation intensity and duration) and the corresponding temporal perfusion dynamics are highlighted.
Theory and methods
Skin tumour and blood perfusion variability
A two-dimensional cylindrical domain was assumed, as shown in , approximating a typical skin tumour of 20 mm diameter and 5 mm depth. The tumour is surrounded by healthy tissue with10 mm radial thickness and a depth of 10 mm. The chosen tumour size corresponds to high risk skin cancers [Citation24,Citation25], which represents a set of good potential candidates for this type of treatment. The nanoparticles were assumed to be uniformly distributed (injected) within the 10 mm radius and 5 mm depth tumour region. is adapted from our previous work and the same two-dimensional domain is continued for the present study [Citation23].
Figure 1. Schematic representing: (A) the tumour region surrounded by healthy skin tissue and (B) a symmetric R–Z cross-section of the tissue. The tumour is surrounded by healthy tissue of 5 mm thickness at the bottom and a 10 mm thick annulus. The symbols R and Z represent radius and depth of tissue, respectively. ΔR and ΔZ represent demarcations of the nodal region. T represents temperature at spatial location R, Z. (Figure reproduced from Soni et al. [Citation23]).
![Figure 1. Schematic representing: (A) the tumour region surrounded by healthy skin tissue and (B) a symmetric R–Z cross-section of the tissue. The tumour is surrounded by healthy tissue of 5 mm thickness at the bottom and a 10 mm thick annulus. The symbols R and Z represent radius and depth of tissue, respectively. ΔR and ΔZ represent demarcations of the nodal region. T represents temperature at spatial location R, Z. (Figure reproduced from Soni et al. [Citation23]).](/cms/asset/47ba2909-2ae0-4f12-8f0c-86b99e815ddc/ihyt_a_1040470_f0001_c.jpg)
The blood perfusion rate among tumours varies widely as compared to the tissue of origin. Depending on several factors, the perfusion rate can either be higher or lower than that of surrounding healthy tissue. A wide range of blood perfusion rates have been reported in the literature for the same tumour type [Citation15,Citation17,Citation18,Citation21,Citation26]. For example, the perfusion rate in a breast tumour can vary by an order of magnitude from 5.3 × 10−3 to 5.3 × 10−2 m3 s−1 m−3 [Citation16]. Further, the tumour blood flow can be modified through injection of certain agents such as hydralazine and phenylephrine [Citation27]. Detailed studies have been presented on the tumour perfusion data [Citation28,Citation29].
Many of the published studies suggest that there is heterogeneous vasculature within a tumour – especially for large tumours. This results in a spatial heterogeneity of the blood perfusion. The literature also suggests that as a tumour grows, it develops a dead (necrotic) cell region at the core [Citation14,Citation28,Citation30–32]. This is characterised by an absence of vasculature in this region and it is called necrotic core. Assuming this is the case, the blood perfusion in the core can be assumed to be negligible. The spatial extent (volume) of the necrotic core depends on the size of tumour.
In light of the wide range and variability of the available blood perfusion data, various perfusion conditions were included to investigate the effect of perfusion on thermal damage for the present study. Since there is so much variation in perfusion rates, this study intends to evaluate thermal damage by simply incorporating a broad range of reported perfusion data. Perfusion conditions for the case of a homogeneously perfused tumour are shown in . For the case of a heterogeneously perfused tumour, spatial variation of perfusion within the tumour is accounted for in the present model (for moderately and highly perfused tumours) by two methods: (1) different perfusion values in the tumour core and periphery, and (2) a necrotic (non-perfused) tumour core. For the tumour sizes considered in this study, the necrotic core is reportedly 40–50% of the tumour volume [Citation30]. Thus, a tumour with a necrotic core of 50% of the total volume is assumed for this study. The perfusion values assumed for a heterogeneously perfused tumour are summarised in . During therapy, the blood perfusion rate varies as a function of vascular stasis (damage to the blood vessels), which is in turn a function of temperature and time.
Table 1. Blood perfusion values for a homogenously perfused tumour and tissue.
Table 2. Spatial variation of blood perfusion (heterogeneously perfused tumour).
Optical interaction of nanoparticle-embedded tumour
Gold nanoshells (GNSs) and nanorods (GNRs) are the most commonly studied particles for nanoparticle-assisted thermal therapy because they are biocompatible and their optical properties can be tuned [Citation33–36]. In the present study GNRs were chosen as they possess up to six times higher absorbance than GNSs [Citation2]. Size selection of the nanoparticles is critical as it governs the conversion of electromagnetic energy to thermal energy. In this study GNRs of 5 nm diameter and an aspect ratio of 3.5 were selected, based on our previous work [Citation6]. This size ensures a much higher value of optical absorption relative to scattering. This is desirable as it will lead to efficient absorption at relatively low irradiation intensity levels. Optical absorption and scattering coefficients of GNRs (embedded with a volume fraction of 0.001% in tumour) were directly obtained from the earlier study [Citation6]. A GNR volume fraction of 0.001% corresponds to a concentration of 0.19 mg/mL. The selected nanoparticle concentration is comparable with other published studies [Citation2,Citation37]. Details on the calculation of optical coefficients are also available in the study [Citation6]. The addition of GNRs to the tumour results in a highly absorbing medium with a maximum absorption coefficient (μa) of 121 cm−1 at the plasmon wavelength (796 nm). This is several orders of magnitude larger than the absorption value for the bare tumour (which is ∼0.06 cm−1[Citation38]). The reduced scattering coefficient (μs’) of a tumour is ∼0.5 times that of healthy tissue (which has a value of 3.25 cm−1 [Citation38,Citation39]). This is due to the fact that a tumour scatters less than healthy tissue due to the lack of a well-differentiated structure [Citation38]. The values of the optical coefficients used herein, along with other salient parameters, are given in .
Table 3. Symbols and values of the various parameters considered in this study.
Since the optical characteristics of a nanoparticle-embedded tumour are a strong function of wavelength, the spectral characteristics of the irradiation must match the optical response of the GNRs. A collimated, near-infrared radiation with a beam diameter of 20 mm (corresponding to the tumour diameter) was selected to uniformly irradiate the tumour surface. The spatial intensity of irradiation was considered with ‘top hat’ or ‘flat’ profile. The spectral emission was taken to be equivalent to a laser with peak irradiance at 796 nm and spectral width of 5 nm. The selected spectral intensity was varied within the range of 0.2–0.3 W cm−2 nm−1 and the corresponding integrated intensity in this spectral band was 1–1.5 W/cm2. This intensity value is optimal for the volume fraction (0.001%) of GNR under consideration [Citation2,Citation6].
For an absorption-dominated medium, the irradiation absorption can be obtained using Beer’s law, as shown in Equation Equation1(1a) . Equation Equation1
(1a) gives accurate results for the condition where NCscaΔZ ≪ 1 [Citation40]. Where, N is the number of nanoparticles per unit volume, Csca is the scattering cross-section of GNR and ΔZ is the discretised thickness or path length. This condition is satisfied for the range of parameters considered in the present study.
(1a)
(1b)
(2)
where,
represents spectral intensity of incident radiation and
denotes spectral intensity attenuated through each layer of thickness ΔZ (as shown in ). The symbols μabs and μsca denote the absorption and scattering coefficients, respectively. Qabs denotes the irradiation absorbed within the tumour. Equation Equation1
(1a) was discretised as a function of wavelength as well as space to obtain the irradiation absorption in each cell of the tumour. The spectral region was divided into wavelength intervals of 1 nm to accurately capture the wavelength-dependent optical absorption.
Thermal damage within the nanoparticle-embedded tumour
Thermal damage to a tissue is a function of temperature and time [Citation41–43]. There are two commonly used models to assess the thermal effects for a tissue. These are (1) a thermal damage (Ω) parameter based on the Arrhenius equation and (2) a thermal dose or cumulative equivalent minutes (CEM). The Arrhenius method, (1), provides a better estimation at higher temperatures, above 48 °C [Citation43]. In our study, thermal damage is evaluated based on the Arrhenius equation. As per the Arrhenius equation, thermal damage over time (duration of exposure, τ) is given by Equation Equation3(3) [Citation43].
(3)
where, Ω, is the thermal damage parameter and it represents the fraction of damaged cells through 1−exp−Ω. When Ω = 1 it signifies that the 64% of the cells are damaged, whereas a value of Ω = 4.6 represents 99.6% cell damage. In Equation Equation3
(3) , the symbol A is a frequency factor and E is the activation energy; collectively these are termed the Arrhenius parameters. Rg is the gas constant, T is the temperature of the tissue and C represents the concentration of cells. The parameters E and A are selected based on tissue type [Citation41]. The Arrhenius parameters considered in this study are mentioned in . Since temperature varies with time, Equation Equation3
(3) was discretised in time to evaluate the thermal damage.
Table 4. Arrhenius parameters considered in this study.
It is well known that as tissue is heated, the blood perfusion rate increases initially and then decreases due to damage to the vasculature. At a certain stage blood perfusion stops completely due to vasculature failure. This phenomenon can be accounted for while evaluating the thermal damage through a parameter termed the ‘degree of stasis’ [Citation21,Citation22]. The degree of stasis or vascular stasis (Θ) represents the damage to the tumour vasculature and is given by Equation Equation4(4) .
(4)
For evaluation of the vascular stasis, the values E and A (given in ) are different than those used for evaluating the thermal damage [Citation22]. In our study, the blood perfusion rate (wb) was varied as a function of the degree of stasis (Θ) through the use of a set of equations given by Equation Equation5(5a) (determined experimentally) [Citation22]. In Equation Equation5
(5a) , wb0 is the initial blood perfusion rate at time t = 0. During therapy, the blood perfusion rate was varied as a function of time through calculation of vascular stasis at each time interval.
(5a)
(5b)
(5c)
(5d)
The overall spatio-temporal thermal damage was then evaluated based on the temperature obtained by solving the Pennes bioheat equation for a two-dimensional cylindrical geometry as given by Equation Equation6(6) . Pennes’ bioheat model is extensively used for these types of heat transfer studies [Citation44,Citation45].
(6)
where, T(r,z,t) is the spatio-temporal temperature. Tc denotes a core body temperature of 37 °C. Qabs denotes the irradiation absorption in the GNR-embedded tumour (evaluated using Equation Equation2
(2) ). Qm denotes the metabolic heat generation rate. wb denotes the blood perfusion. The present study uses Pennes’ bioheat model for evaluating the temperatures. Pennes’ model does not account for the discrete nature of vasculature. Future studies considering this (based on CT data, for example) may be useful for this evolving research area. There are other methods (e.g. the dynamic mode decomposition method) for simulation of the ablation treatment and these successfully employed an extended version of Pennes’ bioheat model [Citation46]. In Equation Equation6
(6) , the blood perfusion term accounts for different perfusion conditions to evaluate its effect on the attained temperature and thus the thermal damage during treatment. The meanings of the other symbols in Equation Equation6
(6) and their respective values are given in . Equation Equation6
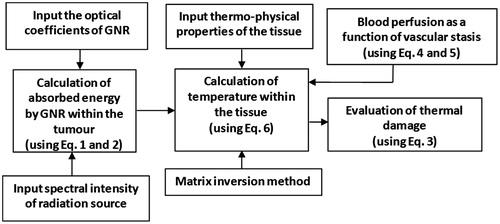
(6) was discretised using the finite difference implicit scheme. The nodal equations were generated by applying an energy balance for each nodal region [Citation47]. Unknown temperatures were evaluated through the matrix inversion method implemented in MATLAB. An ambient natural convection boundary (10 W/cm2K, 25 °C) was considered at the top surface and a constant temperature condition of 37 °C was assumed at the other boundaries. A temperature of 35 °C was set as the initial condition for evaluating the spatio-temporal temperature field. The physical domain was discretised to radial and axial elements totalling to 81 × 41, respectively. For higher elements (101 × 51), the grid dependency of the results obtained was less than 0.1 °C. So a grid size of 81 × 41 was selected for evaluating the results. It is well known that a temperature of ∼50–60 °C is sufficient to thermally damage tissue [Citation48]. The thermal damage parameter (Ω) was restricted to 4.6 (corresponding to 99.6% cell damage) because further increasing this parameter has little significance [Citation20,Citation43]. summarises the steps followed for evaluating the influence of blood perfusion on thermal damage.
The numerical model developed was validated through comparison with published studies [Citation1,Citation2] as detailed in our earlier work [Citation6].
Results and discussion
The spatio-temporal thermal ablation temperature and thermal damage evaluated for different cases of perfusion rates are presented and discussed in this section. At the end of this section we also highlight the temporal perfusion dynamics for the defined (ideal) therapeutic parameters.
Homogeneously perfused tumour
The temperatures as well as thermal damage (Ω) were evaluated considering the perfusion values (as listed in ) for the conditions of constant blood perfusion and for variable perfusion (e.g. temporal variation based on the vascular stasis). The blood perfusion rate within the tumour was assumed homogeneous for obtaining the following results.
Low perfusion (almost equal perfusion within tumour and tissue)
For the low perfusion rates within the tumour and the surrounding healthy tissue, show the contours of the evaluated ablation temperature and the spatial profile of thermal damage during nanoparticle-assisted thermal therapy. Propagation of the thermal damage zone during irradiation is shown in .
Figure 3. (A) Contour plot for thermal ablation temperature, (B) spatial profile of the thermal damage, at the end of irradiation duration of 105 s. (c) Contours for thermal damage zone (Ω = 4.6) at various time instants during irradiation. Irradiation intensity is 1 W/cm2 for 105 s. GNR volume fraction is 0.001%. Perfusion data is as per for the case of low perfusion. Constant blood perfusion is assumed (i.e. not varying with time).
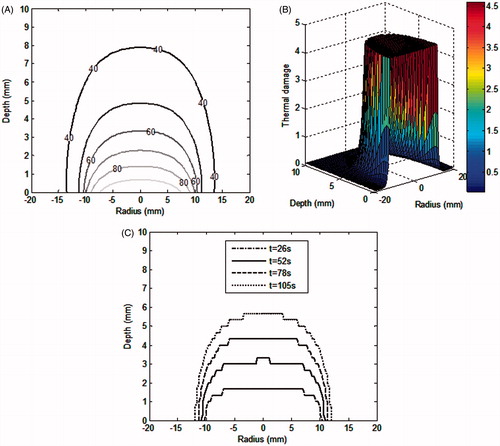
It can be seen from that the desired thermal ablation temperature is attained within the tumour region. The corresponding thermal damage zone, as shown in , covers the tumour region. shows that with irradiation, the thermal damage zone propagates towards the deeper tumour region. At higher depths, the thermal damage zone narrows down along radial direction. This is due to the fact that during therapy, the blood perfusion at the tumour–healthy tissue boundary increases resulting in higher heat loss and hence lowers the ablation temperatures in this region.
Moderate perfusion
The temperatures as well as thermal damage were also evaluated considering higher perfusion values within the tumour as compared to surrounding healthy tissue. The thermal damage zone (Ω = 4.6) for the three different perfusion cases (as mentioned in ) are collectively shown in . show the results for constant perfusion and for variable perfusion (temporal variation as a function of vascular stasis), respectively.
Figure 4. Contours of thermal damage (Ω = 4.6) for three different blood perfusion values considering (A) constant blood perfusion i.e. not varying with time, (B) blood perfusion varying with vascular stasis. Irradiation intensity is 1 W/cm2 for 105 s. GNR volume fraction is 0.001%. Perfusion rates are as per .
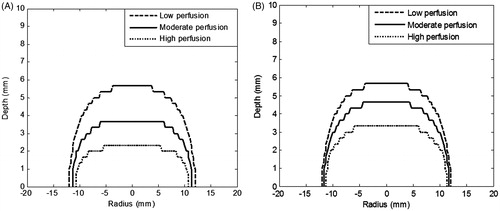
It can be seen from that for the ‘low perfusion case’, the spatial extent of thermal damage was not influenced by incorporating the vascular stasis-based variable perfusion. For these perfusion rates, the calculations showed that the temperature obtained at 5 mm depth within the tumour, T(0,5) was the same (49.2 °C) as in the constant and vascular stasis-based perfusion cases. Also, the surface temperatures were not significantly altered. So for the low perfusion rates it may not be necessary to incorporate a temporal variation of perfusion. It is to be noted that the therapy duration is quite short (∼2 min as opposed to 30–120 min in the case of conventional hyperthermia). So, for low perfusion rates, shorter therapeutic duration does not allow a significant loss of thermal energy due to blood flow (time scales of the order of 1 min). On the contrary, one study showed that blood flow may play important role in the shorter time scales of ∼8 s for ultrasonic lesion formation [Citation49]. While this finding is not supported by our model (for low perfusion rates), more insight on this may be obtained through future studies considering the discrete nature of the vasculature.
For the moderate perfusion case, e.g. comparing , it can be seen that the thermal damage spreads by 0.9 mm to a depth of 4.6 mm. Overall increase in the thermal damage zone is 25%. This means that vascular stasis aids the penetration of thermal damage to the deeper tumour region. So for moderate perfusion rates, there is a need to incorporate temporal variation of blood perfusion to get exact estimates of the thermal damage zone. But still the thermal damage does not cover the full depth of the tumour, so the tumour needs to be irradiated for a longer duration.
High perfusion
In this section, high perfusion rates (matching the highest reported values in literature) were assumed for both the tumour and surrounding healthy tissue. The perfusion rate in healthy tissue is one order of magnitude higher than that assumed in the Moderate perfusion section above. Using these high perfusion rates (as mentioned in ), the thermal damage was evaluated for an irradiation duration of 105 s. The evaluated thermal damage zone for this case is included in .
Comparing , it can be seen that for the high perfusion case, the incorporation of the variable perfusion extends the thermal damage zone by 1 mm, i.e. penetration of thermal damage down to a 3.3 mm depth of tumour. In this case the thermal damage zone would be under-predicted by 52% if we consider a constant perfusion rate during the therapy. This is due to the fact that vascular stasis shuts off blood flow in the region and subsequently there is no heat loss due to blood flow. Overall, for moderate and high perfusion cases the thermal damage was extended to a depth of 4.6 mm and 3.3 mm, respectively. This shows that temporal variation in perfusion needs to be considered for moderate and high perfusion. But for fixed irradiation parameters and nanoparticle volume fractions, higher perfusion rates still result in significant heat loss from the tumour, and thus incomplete damage. So for moderate and high perfusion rates, the tumour needs to be irradiated for a longer duration to extend the thermal damage into the deeper tumour region (to a depth of 5 mm). Upon increasing the irradiation duration (of a fixed intensity) to 150 s (an increase of ∼1.5×), the thermal damage zones attained are shown in . The irradiation duration was selected to avoid a tumour surface temperature of more than 100 °C.
Figure 5. Contours of thermal damage zone (Ω = 4.6) within the homogeneously perfused tumour and tissue considering (A) constant blood perfusion, i.e. not varying with time, (B) blood perfusion as a function of vascular stasis. Irradiation intensity of 1 W/cm2 for 150 s. GNR volume fraction is 0.001%. Perfusion rates are assumed as per .
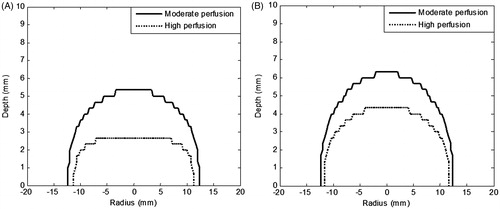
It can be seen from that for moderate perfusion the thermal damage zone covers the entire tumour depth, whereas for high perfusion the thermal damage (Ω = 4.6) was still restricted to a 4.2 mm depth which is not enough to completely damage the tumour. So there is a need to optimise for higher irradiation intensity for this case.
Importantly, the results discussed show marked variation in the thermal damage for the constant versus varying perfusion (temporal variation). The results also indicate that (for the high perfusion case) higher irradiation duration of 150 s (for a fixed irradiation intensity of 1 W/cm2) does not lead to the desired spread of thermal damage to the tumour depth of 5 mm.
Heterogeneously perfused tumour (spatial variation of blood perfusion within tumour)
For the results discussed in the previous subsection, a homogenous blood perfusion rate was assumed within the tumour. As mentioned earlier, there is a spatial variation of the perfusion in large tumours. So, considering the perfusion data in , the temperature field and thermal damage were evaluated for a moderately and highly perfused tumour (with and without necrotic core). In the present numerical model we have assumed a uniform concentration of GNRs within the whole tumour. The presence/absence of nanoparticles in the core depends on the method opted for delivering the nanoparticles to the tumour. Nanoparticles delivered through an intravenous method tend to reside around the vascularised region of the tumour, whereas nanoparticles delivered through skilled local/interstitial injection can potentially deliver a uniform concentration into the injected area. This distribution role of nanoparticle concentrations was highlighted in our earlier study [Citation23]. The temperature contours attained for a highly perfused tumour with a necrotic core are shown in . show the results for irradiation duration of 105 s and 150 s, respectively. The corresponding results for thermal damage are shown in . It was found that upon increasing the irradiation duration to 150 s, the surface temperature T(0,0) just reached 100 °C. Thus, the irradiation duration was restricted to 150 s.
Figure 6. Contour plot of temperature for highly perfused heterogeneous tumour with necrotic core. Irradiation intensity is 1 W/cm2 for duration of (A) 105 s, and (B) 150 s. GNR volume fraction is 0.001%. Vascular stasis-based perfusion variation is assumed . Perfusion data is for highly perfused tumour as per .
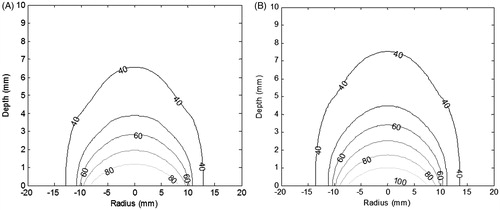
Figure 7. Contours of thermal damage zone (Ω = 4.6) for a heterogeneously perfused tumour for irradiation duration of (A) 105 s, and (B) 150 s. Irradiation intensity is 1 W/cm2 and GNR volume fraction is 0.001%. The blood perfusion rate was varied as a function of vascular stasis. Perfusion rates are as per .
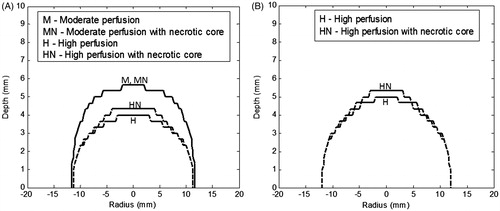
show that with higher irradiation duration there is a slight gain in temperature (at a depth of 5 mm). It can be seen that the 50 °C temperature contour is extended to a depth of 4.3 mm. But as seen from , there is substantial gain in the thermal damage zone due to longer exposure of the tumour to the temperatures (time is also a factor for thermal damage). shows that by increasing the irradiation duration to 150 s for a highly perfused heterogeneous tumour, the thermal damage zone spreads well to ∼5-mm depth.
By comparing the temperature contours for a moderately perfused heterogeneous tumour () with the homogeneously perfused tumour (low perfusion case, , where perfusions are of the same order of magnitude), the spatial variation of the perfusion rate within the tumour did not significantly influence the thermal damage along the depth as well as the radius. So incorporating the perfusion heterogeneity for such perfusion rates does not alter the thermal damage zone. Also, it can be seen from that for a moderately perfused tumour there is nil variation in thermal damage upon incorporating perfusion with and without necrotic core.
shows that the spatial heterogeneity of perfusion plays an important role for high perfusion rates. It is inferred from that for a highly perfused tumour with a necrotic core the thermal damage penetrates deeper due to the absence of perfusion at the tumour core, but that it drops sharply at the tumour periphery.
Therapeutic parameters versus perfusion dynamics
The results discussed above imply that the spatio-temporal perfusion dynamics (during the therapy) influence the rate of thermal energy stored within the tumour as well as the rate at which the heat transfer occurs to the blood (Qblood). So, for a given tumour tissue perfusion dataset, the external input power should overcome the Qblood as per perfusion dynamics. Only then can ablation of the whole tumour be achieved. show the temporal variation in the thermal energy storage and heat loss rates at various depths within the tumour (considering vascular stasis-based perfusion). These are plotted for moderately and highly perfused, heterogeneous, non-necrotic tumours (data as per Table 2), respectively.
Figure 8. Temporal variation in the heat stored within the heterogeneously perfused tumour and heat lost due to blood perfusion for (A) moderately perfused tumour, (B) highly perfused tumour. Irradiation intensity is 1 W/cm2 for irradiation duration of 105 s and GNR volume fraction is 0.001%. Vascular stasis-based perfusion is assumed. Perfusion rates are considered as per . Please note the different y-scale magnitude for ‘heat loss due to blood’ plots.
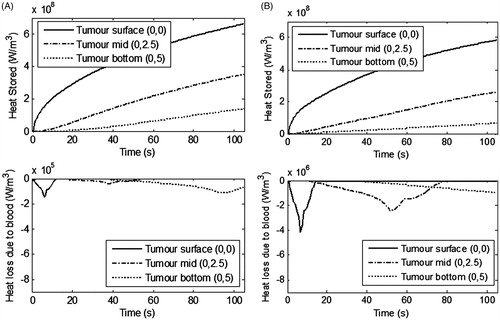
shows that for a moderately perfused tumour the thermal energy stored within the tumour is sufficient for thermal damage. Heat transfer to the blood flow is minimal and thus the desired thermal damage could be attained within the tumour for an irradiation duration of 105 s, but for the highly perfused tumour (as seen from ), the peak heat loss due to blood flow is almost 28 times higher at the tumour surface. It can also be seen that at the mid region of the tumour the heat loss due to blood is higher and continues for longer time-scales compared to that for the moderately perfused tumour. Perfusion ceases at some time point due to complete damage of the blood vessels in this region. This considerable heat loss due to blood flow, for the same irradiation parameters (1 W/cm2 for 105 s), restricts the thermal damage to a partial region of highly perfused tumour. A greater spatial extent of thermal damage can be achieved by either increasing the irradiation intensity, duration, or volume fraction of the nanoparticles. A recent study showed that for nanoparticle volume fractions higher than 0.001%, there is no significant increase in penetration of therapy deeper within the tumour [Citation23]. So keeping a fixed dose of nanoparticles, the other parameters, i.e. irradiation intensity and duration, can be enhanced to attain the desired thermal damage. For the present case, higher irradiation duration of 150 s is used to increase the depth of thermal damage in the tumour.
For the therapeutic parameters considered here, the temporal changes in the ‘degree of stasis’ and blood perfusion rate were also highlighted for the moderately and highly perfused tumours. These plots are shown in .
Figure 9. Temporal variation in the degree of vascular stasis and corresponding blood perfusion within the heterogeneously perfused tumour for (A) moderately perfused tumour, (B) highly perfused tumour. Irradiation intensity is 1 W/cm2 for irradiation duration of 105 s and GNR volume fraction is 0.001%. Perfusion rates are as per . Please note the different y-scale magnitude for ‘blood perfusion’ plots.
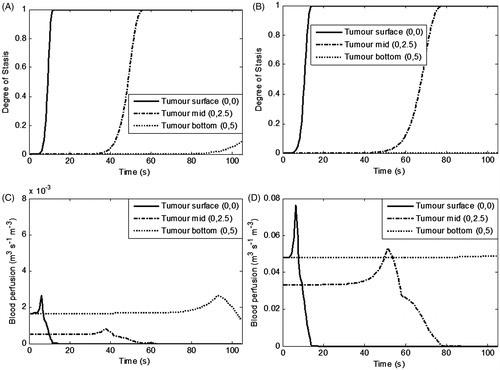
It can be seen from that at the tumour surface stasis occurs almost instantaneously, whereas at the tumour mid region stasis duration is influenced by the blood perfusion rate. shows that at the mid region for the highly perfused tumour tissue, the stasis completed at longer time scales (30 s) as compared to the moderate perfusion region (22 s). This is expected considering that during therapy the nanoparticles at the surface absorb a major part of the irradiation intensity. Stasis at the tumour bottom is negligible for a highly perfused tumour because, as discussed above, for 105 s irradiation, thermal ablation conditions are not attained in this region.
Regarding the corresponding blood perfusion variation, it can be seen from that at the tumour surface the blood perfusion rises sharply (1.5 and 1.7 times for moderately and highly perfused tumours respectively) and then drops to zero due to complete vascular stasis. Similarly, at the mid region of the tumour, there is a rise and drop in the perfusion, but this occurs at longer time scales. For the moderately perfused tumour, at the bottom region, perfusion rises due to a rise in the temperature in this region. But for the highly perfused tumour (bottom region), there is no change in the perfusion rate because the irradiation duration of 105 s was not enough to attain thermal damage at a 5-mm tumour depth. This is the why the irradiation duration was enhanced to 150 s (), which can provide the desired thermal damage zone for the highly perfused heterogeneous tumour. For the tumour size and perfusion conditions under consideration, the ideal therapeutic parameters were found to be irradiation intensity of 1 W/cm2, and irradiation duration of 105–150 s for a nanoparticle volume fraction of 0.001%.
During hyperthermia and thermal ablation, the systemic temperature may increase. The magnitude of this increase depends on the treatment volume to which the energy is applied and on the methods to cool the patient [Citation50]. It has been reported that for regional hyperthermia, a modest increase in the systemic temperature could counteract the increased cooling by localised blood perfusion, depending on the relative time scales [Citation51]. However, systematic temperature increase would take several minutes to 1 h, which is unlikely to affect nanoparticle-assisted thermal therapy which takes in the order of ∼2 min. This issue may be investigated in detail in future studies.
Conclusions
In this study the role of the variability in blood perfusion for tumours and the surrounding healthy tissue was investigated during nanoparticle-assisted thermal therapy. Spatio-temporal variability of blood perfusion for various perfusion conditions was considered and the corresponding thermal damage zones were evaluated to understand the influence of blood perfusion.
The results show that for low perfusion rates the temporal variation in blood perfusion (during a therapy duration of ∼2 min) does not critically influence thermal damage. In the case of moderately and highly perfused tumours a temporal variation in the blood perfusion rate extends the thermal damage zone by 25–52% compared to the constant perfusion rate. Regarding the tumour perfusion heterogeneity (necrotic versus non-necrotic core), the spatial variation of the perfusion within a moderately perfused tumour does not alter the thermal damage zone. For highly perfused heterogeneous tumours, however, the necrotic core enables the extent of the thermal damage zone to reach deeper into the tumour region. For the tumour size and perfusion conditions considered in this study, the ideal therapeutic parameters were found to be an irradiation intensity of 1 W/cm2 for a duration of 105–150 s with a uniform nanoparticle volume fraction of 0.001% within the tumour.
In conclusion, for low perfusion rates nanoparticle-assisted thermal therapy is relatively insensitive to temporal variation in the perfusion rate during therapy (due to the shorter therapeutic duration of ∼2 min as compared to the 30–120 min of conventional hyperthermia). For moderately and highly perfused tumours, the assumption of constant perfusion (during therapy) under-predicts the thermal damage zone. So relying on constant perfusion-based thermal damage results can lead to significant damage of surrounding healthy tissue due to under-prediction of the thermal damage zone. For effective therapy, the therapeutic parameters need to be selected based on perfusion dynamics to overcome the heat loss due to blood perfusion.
Declaration of interest
S.S. and H.T. wish to acknowledge the support provided by the School of Mechanical, Materials and Energy Engineering at the Indian Institute of Technology, Ropar. S.S. also acknowledges the support of CSIR - Central Scientific Instruments Organisation, Chandigarh. R.A.T. acknowledges the University of New South Wales Early Career Researcher (ECR) faculty scheme and the School of Mechanical and Manufacturing Engineering (MECH) seed research funding scheme for support. The authors alone are responsible for the content and writing of the paper.
References
- Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumours under magnetic resonance guidance. Proc Natl Acad Sci USA 2003;100:13549–54
- Maltzahn GV, Park J, Agrawal A, Bandaru NK, Das SK, Sailor MJ, et al. Computationally guided photothermal tumour therapy using long-circulating gold nanorod antennas. Cancer Res 2009;69:3892–900
- Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011;7:169–83
- Kannadorai RK, Liu Q. Optimization in interstitial plasmonic photothermal therapy for treatment planning. Med Phys 2013;40:103301
- Ghosh S, Gupta DD, Chakraborty S, Das SK. Superparamagnetic nanoparticle-assisted hyperthermia and cooling protocol for optimum damage of internal carcinoma using computational predictive model. Heat Mass Transfer 2013;49:1217–29
- Soni S, Tyagi H, Taylor RA, Kumar A. Role of optical coefficients and healthy tissue-sparing characteristics in gold nanorod-assisted thermal therapy. Int J Hyperthermia 2013;29:87–97
- Mobley J, Vo-Dinh T. Optical properties of tissues. In: Vo-Dinh T, ed. Biomedical Photonics Handbook. Boca Raton, FL: CRC Press, 2003. 2-1--2-75
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993;9:51–68
- Jordan A. Letter to the editor, Hyperthermia classic commentary: ‘Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia’ by Andreas Jordan et al., International Journal of Hyperthermia, 1993;9:51–68. Int J Hyperthermia 2009;25:512–16
- Jordan A, Scholz R, Maier-Hauff K, Johannsen M, Wust P, Nadobny J, et al. Presentation of a new magnetic field therapy system for the treatment of human solid tumours with magnetic fluid hyperthermia. J Magn Magn Mater 2001;225:118–26
- Stolwijk JA. Mathematical models of thermal regulation. Ann N Y Acad Sci 1980;335:98–106
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumours. Nat Rev Clin Oncol 2010;7:653–64
- Dreher MR, Liu W, Michelich CR, Dewhirst MW, Yuan F, Chilkoti A. Tumour vascular permeability, accumulation and penetration of macromolecular drug carriers. J Natl Cancer Inst 2006;98:335–44
- Kuszyk BS, Corl FM, Franano FN, Bluemke DA, Hofmann LV, Fortman BJ, et al. Tumour transport physiology: Implications for imaging and imaging guided therapy. Am J Roentgenol 2001;177:747–53
- Fujita S, Tamazawa M, Kuroda K. Effects of blood perfusion rate on the optimization of RF-capacitive hyperthermia. IEEE Trans Biomed Eng 1998;45:1182–6
- Vaupel P. Pathophysiology of solid tumours. In: Molls M, Vaupel P, Nieder C, Anscher MS, editors. The impact of tumour biology on cancer treatment and multidisciplinary strategies. Berlin: Springer Verlag, 2009. 51--92
- Wilson CBJH, Lammertsma AA, McKenzie CG, Sikora K, Jones T. Measurements of blood flow and exchanging water space in breast tumours using positron emission tomography: A rapid and noninvasive dynamic method. Cancer Res 1992;52:1592–7
- Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Charlop A, Lawton TJ, et al. Blood flow and metabolism in locally advanced breast cancer: Relationship to response to therapy. J Nucl Med 2002;43:500–9
- Baish JW. Microvascular heat transfer. In: Bronzino JD, editor. The Biomedical Engineering Handbook. 2nd ed. Boca Raton, FL: CRC Press, 2000. 98-1--98-14
- Prakash P, Diederich CJ. Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: Implications for treatment planning, monitoring and control. Int J Hyperthermia 2012;28:69–86
- Schutt DJ, Haemmerich D. Effects of variation in perfusion rates and of perfusion models in computational models of radio frequency tumour ablation. Med Phys 2008;35:3462–70
- He X, McGee S, Coad JE, Schmidlin F, Iaizzo PA, Swanlund DJ, et al. Investigation of the thermal and tissue injury behaviour in microwave thermal therapy using a porcine kidney model. Int J Hyperthermia 2004;20:567–93
- Soni S, Tyagi H, Taylor RA, Kumar A. Investigation on nanoparticle distribution for thermal ablation of a tumour subjected to nanoparticle assisted thermal therapy. J Therm Biol 2014;43:70–80
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 1970;172:902–8
- Samarasinghe V, Madan V. Nonmelanoma skin cancer. J Cutan Aesthet Surg 2012;5:3–10
- Bagaria HG, Johnson DT. Transient solution to the bioheat equation and optimization for magnetic fluid hyperthermia treatment. Int J Hyperthermia 2005;21:57–75
- Wu H, Exner AA, Krupka TM, Weinberg BD, Haaga JR. Vasomodulation of tumour blood flow: Effect on perfusion and thermal ablation size. Ann Biomed Eng 2009;37:552–64
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumours: A review. Cancer Res 1989;49:6449–65
- Jain RK, Ward-Hartley K. Tumour blood flow-characterization, modifications and role in hyperthermia. IEEE Trans Son Ultrason 1984;SU-31:504–26
- Hilmas DE, Gillette EL. Morphometric analyses of the microvasculature of tumours during growth and after X-irradiation. Cancer 1974;33:103–10
- Baxter LT, Jain RK. Transport of fluid and macromolecules in tumours. II. Role of heterogeneous perfusion and lymphatics. Microvasc Res 1990;40:246–63
- Jain RK. Transport of molecules, particles, and cells in solid tumours. Annu Rev Biomed Eng 1999;01:241–63
- Jain PK, Lee KS, Ivan HE, Mostafa AE. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J Phys Chem B 2006;110:7238–48
- Hewakuruppu YL, Dombrovsky LA, Timchenko V, Yeoh GH, Jiang XC, Taylor RA. Optimisation of metallic nanoshell suspensions for radiation experiments. Int J Trans Phenomena 2013;13:233–44
- Hewakuruppu YL, Dombrovsky LA, Chen C, Timchenko V, Jiang X, Baek S, et al. Plasmonic ‘pump–probe’ method to study semi-transparent nanofluids. Appl Opt 2013;52:6041–50
- Taylor RA, Wong JK, Baek S, Hewakuruppu YL, Jiang X, Chen C, et al. Nanoparticle-assisted heating utilizing a low-cost white light source. J Nanotechnol Eng Med 2013;4:041005
- Didychuk CL, Ephrat P, Chamson-Reig A, Jacques SL, Carson JJL. Depth of photothermal conversion of gold nanorods embedded in a tissue-like phantom. Nanotechnology 2009;20:195102
- Tromberg BJ, Shah N, Lanning R, Cerussi A, Espinoza J, Pham T, et al. Non invasive in vivo characterization of breast tumours using photon migration spectroscopy. Neoplasia 2000;2:26–40
- Zonios G, Dimou A. Light scattering spectroscopy of human skin in vivo. Optics Express 2009;17:1256–67
- Gaponenko SV. Introduction to Nanophotonics. New York: Cambridge University Press, 2010
- Wright NT. On a relationship between the Arrhenius parameters from thermal damage studies. J Biomech Eng 2003;125:300–4
- Pearce J. Mathematical models of laser-induced tissue thermal damage. Int J Hyperthermia 2011;27:741–50
- Pearce JA. Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia 2013;29:262–80
- Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol 1948;1:93–122
- Wissler EH. Pennes’ 1948 paper revisited. J Appl Physiol 1998;85:35–41
- Bourantas GC, Ghommem M, Kagadis GC, Katsanos K, Loukopoulos VC, Burganos VN, Nikiforidis GC. Real time tumour ablation simulation based on the dynamic mode decomposition method. Med Phys 2014;41:053301
- Incropera FP, Dewitt DP. Fundamentals of Heat and Mass Transfer. 5th ed. New Delhi: Wiley India, 2009
- Stauffer PR, Goldberg SN. Introduction: Thermal ablation therapy. Int J Hyperthermia 2004;20:671–7
- Kolios MC, Sherar MD, Hunt JW. Blood flow cooling and ultrasonic lesion formation. Med Phys 1996;23:1287–98
- Zee JV. Heating the patient: A promising approach. Ann Oncol 2002;13:1173–84
- De Leeuw AA, Van Vulpen M, Van De Kamer JB, Wárlám-Rodenhuis CC, Lagendijk JJ. Increasing the systemic temperature during regional hyperthermia: Effect of a cooling strategy on tumour temperatures and side-effects. Int J Hyperthermia 2003;19:655–63