Abstract
Objective: The aim of this study was to compare high-intensity focused ultrasound (HIFU) treatment for type I and type II submucosal fibroids. Materials and methods: From October 2011 to October 2013, 55 patients with submucosal fibroids were enrolled in this study. Based on submucosal fibroid classification, 27 patients were grouped as type I submucosal fibroids, and 28 patients were classified as type II submucosal fibroids. All patients received HIFU treatment and completed 1-, 6-, and 12-month follow-ups. Adverse effects were recorded. Results: There were no significant differences in the baseline characteristics between the two groups (p > 0.05). Using similar sonication power, sonication time, and acoustic energy, the non-perfused volume (NPV) ratio was 83.0 ± 17.3% in the type I group, and 92.0 ± 9.5% in the type II group. All the patients tolerated the procedure well, and no serious adverse events occurred. During the follow-up intervals, the treated fibroids shrank and fibroid-related symptoms were relieved. No other reinterventional procedures were performed during the follow-up period. Conclusion: Based on our results with a small number of subjects, HIFU is suitable for both type I and type II submucosal fibroids. It seems that type II submucosal fibroids are more sensitive to HIFU ablation. Future studies with larger sample sizes and longer follow-up times to investigate the long-term results, including long-term symptom relief, pregnancy outcomes, and the recurrence rate as well as the reintervention rate are needed.
Introduction
Submucosal fibroids that grow into the uterine cavity with the endometrium covering on the surface distort the endometrial cavity and typically cause heavy or irregular menstrual bleeding in premenopausal women [Citation1]. They have also been identified as a potential cause of infertility, preterm delivery, early miscarriage and secondary anaemia [Citation2]. Based on the relationship between fibroids and endometrial cavity, the European Society of Gynaecological Endoscopy has classified submucosal fibroids under three categories: type 0 if the fibroid is entirely within the endometrial cavity; type I if ≥50% of the fibroid body is in the endometrial cavity; and type II if <50% of the fibroid body is in the endometrial cavity [Citation3].
With the development of endoscopy, hysteroscopic myomectomy as a minimally invasive technique has made fibroids accessible and resectable from the inner surface of the uterus. Although hysteroscopic myomectomy is currently considered to be the treatment of choice for submucosal fibroids, its efficacy and safety are closely related to the size and type of submucosal fibroids [Citation4]. It is well accepted by clinical practitioners that hysteroscopic myomectomy is suitable for type 0 submucosal fibroids with a size preferably smaller than 5 cm in diameter, because these fibroids could be completely removed with limited damage to the uterus and the endometrium [Citation5]. However, in the cases of type I and type II fibroids, low complete removal rates, high complication rates, and high recurrence rates have limited the application of hysteroscopic myomectomy [Citation6,Citation7].
Uterine artery embolisation (UAE) is another effective minimally invasive conservative therapy for the treatment of symptomatic submucosal fibroids. However, early post-embolisation syndrome including pain, nausea, a flu-like illness, and mild pyrexia, and late complications including chronic vaginal discharge, adverse changes in sexual function and temporary amenorrhea have also limited the application [Citation8,Citation9].
Over the last 10 years, many studies have demonstrated that high-intensity focused ultrasound (HIFU) ablation, as a novel non-invasive technique, is a safe and effective method for the management of uterine fibroids [Citation10–13]. Based on the magnetic resonance imaging (MRI) T2 signal intensity, uterine fibroids were classified as hyperintense, isointense, or hypointense. Funaki et al. [Citation14] found that MR-guided focused ultrasound surgery (MRgFUS) is an appropriate option for isointense and hypointense uterine fibroids. Zhao et al. [Citation15] further found that heterogeneous and markedly homogeneous hyperintense fibroids were suitable for ultrasound-guided HIFU (USgHIFU), and only the slightly homogeneous hyperintense fibroids should be excluded. HIFU ablation is capable of causing coagulative necrosis at a precise focal point within the body by using an external ultrasonic energy source to penetrate through intact skin [Citation16]. Since the ablation range can be precisely controlled, the application of HIFU treatment on submucosal fibroids is feasible, due to it being unlikely to damage the neighbouring uterine endometrium. Jung et al. [Citation17] have reported a case of successful HIFU treatment for disconnecting symptomatic intracavitary submucosal uterine fibroids without the need for any additional invasive procedures. Wang et al. [Citation18] also demonstrated that HIFU is safe and effective in treating submucosal fibroids. However, to the best of our knowledge, no comparison study of HIFU treatment for type I and type II submucosal fibroids was performed. Therefore, in our study we compared the HIFU treatment results of type I and type II submucosal fibroids, which we believe can provide more evidence for alternative non-invasive therapies of submucosal fibroids for clinical practitioners.
Materials and methods
Patients
This prospective study was approved by the ethics committees at our institutions. Every patient signed an informed consent before HIFU treatment.
The inclusion and exclusion criteria were as described in previous studies [Citation10,Citation13]. Briefly, the inclusion criteria were (1) the diagnosis of submucosal fibroids was made by clinical examination, ultrasound (US) and MRI, (2) patients were more than 18 years old and in premenopausal status, and (3) patients had dysmenorrhoea, menorrhagia and/or anaemia and required treatment. Exclusion criteria included (1) type 0 submucosal fibroid, (2) multiple fibroids, and (3) patients with suspected or confirmed uterine malignancies.
From October 2011 to October 2013, 55 eligible patients were enrolled in this study. According to submucosal fibroid classification, the submucosal fibroids in 27 patients were classified as type I and the submucosal fibroids in 28 patients were classified as type II.
Ultrasound and MR imaging
All the patients were examined beforehand by Color Doppler ultrasound (Esaote, Genoa, Italy), and at 1 month, 6 months and 12 months after HIFU treatment to observe the size of the fibroids and monitor blood flow distribution.
Contrast-enhanced MRI (Siemens Healthcare, Erlangen, Germany) was also conducted on every patient before HIFU treatment to determine the appropriate treatment location and margins, as well as at 1, 6, and 12 months after HIFU treatment for follow-up (). Non-perfused volume (NPV), defined as the area not enhanced in the contrast-enhanced MR imaging, was measured post-HIFU treatment. All US and MR images were assessed independently by two senior radiologists.
Pre-treatment preparation
Patients followed strict diet preparations 3 days before HIFU ablation. A bland diet was required for patients on the first day, followed by semi-liquid and liquid food without milk on the second and third day, respectively. Fasting 12 h pre-treatment and enema on the morning of the treatment day were also required. Skin preparation included shaving of the hair from the umbilicus to the upper margin of the pubic symphysis, degreasing, and degassing with 75% ethanol. A urinary catheter was used to release urine and was refilled with saline for the purpose of improving the therapeutic acoustic pathway. Degassed water balloons with different sizes and tensions to push bowels away from the acoustic pathway for intestinal safety were also prepared for each patient.
HIFU ablation
HIFU treatment was performed using an USgHIFU system (JC200, Haifu® Technology, Chongqing, China). Therapeutic focused ultrasound energy was produced with a 20-cm diameter transducer with a focal length of 15 cm. Patients were carefully positioned prone on the HIFU table, with the anterior abdominal wall placed in contact with degassed water. HIFU treatment was performed under intravenous conscious sedation. Patients received 50–400 μg of fentanyl (Yichang Humanwell Pharmaceutical, Yichang, China) and 1–4 mg of midazolam hydrochloride (Jiangsu Nhwa Pharmaceutical, Xuzhou, China) to minimise discomfort and to prevent unnecessary body movement.
Sagittal ultrasound scanning mode was used for observing the positional relationship between the uterine lesion and the bladder. The applied ultrasound had a frequency of 1.0 MHz, an oval focal point 3 mm in width and 8 mm in length, a pulse with a sonication time of 1–2 s, and an interval time of 3–6 s. Acoustic power of 350–400 W was used. When greyscale changes occurred and sufficient thermal dose was reached, the focal point was moved to the next spot to achieve complete ablation of the planned treatment volume. The distance was 5 mm between the spots, lines and layers. All HIFU treatments were administered with a margin of 5 mm from the fibroid surface in order to prevent thermal damage to the adjacent endometrium and myometrial wall.
An ambulatory electrocardiogram (ECG) monitor was used to evaluate the respiration, heart rate, blood pressure and oxygen saturation level. Contrast-enhanced ultrasound using a microbubble agent (SonoVue, Bracco, Milan, Italy) was performed before and immediately after HIFU treatment to evaluate therapeutic results. In brief, 25 mg SonoVue was reconstituted with 5 mL of normal saline and 2 mL of SonoVue (10 mg) was injected intravenously each time. The contrast-enhanced ultrasound results were analysed independently by two experienced radiologists (). Patients were discharged from the HIFU units 30 min after HIFU treatment.
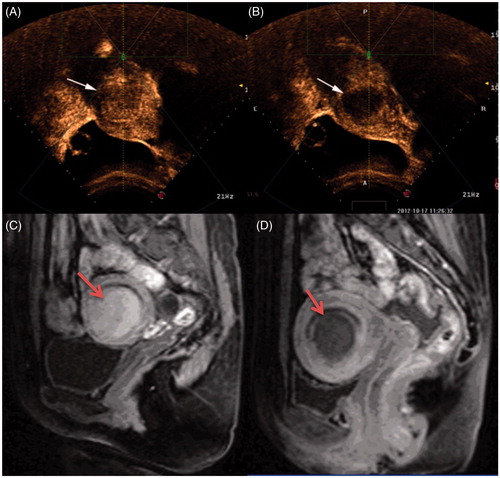
Figure 1. Contrast-enhanced ultrasound images and MR images obtained from a patient with type I submucosal fibroid. (A) Pre-procedure contrast-enhanced ultrasound image shows a submucosal fibroid (arrow). (B) Contrast-enhanced ultrasound image obtained immediately after HIFU shows the non-perfused area (arrow). (C) Pre-procedure MRI shows a 4.5 × 4.3 × 4.0 cm submucosal fibroid located at the anterior wall of the uterus (arrow). (D) Contrast-enhanced MRI obtained 1 day after HIFU shows the fractional ablation was 90% (arrow).
Safety assessments
Incidences of any HIFU-related adverse events, including any discomfort on skin, lower abdominal pain, leg pain, sciatic or buttock pain, and vaginal discharge or bleeding were recorded.
Follow-up
Patients were followed up at 1-month, 6-month and 12-month intervals after they were discharged from the hospital. Clinical assessment was performed to compare the changes of fibroid-related symptoms. Ultrasound and MRI was used to evaluate the size changes of the ablated submucosal fibroids.
Statistical analysis
All data were analysed with SPSS 17.0 (Chicago, IL, USA). Data were recorded as means ± standard deviations (SD). The Wilcoxon test and chi-squared test were applied for the analysis of the quantitative and enumerated data, respectively. ANOVA analysis was also applied when comparing variables at different time points of the follow-up period within and between two groups. A p value of less than 0.05 was considered to indicate a significant difference.
Results
Demographic characteristics of patients with submucosal fibroids
shows the average age was 40.9 ± 5.4 years for patients with type I submucosal fibroids and 41.9 ± 6.1 years for patients with type II submucosal fibroids. The mean body mass indexes (BMIs) of patients with type I and type II submucosal fibroids were 22.4 ± 3.8 kg/m2 and 23.3 ± 3.1 kg/m2, respectively. Based on MRI, the average largest fibroid diameter and volume were 4.2 ± 1.9 cm (range 1.8–9.0 cm) and 47.8 ± 60.4 cm3 (range 1.5–263.7 cm3) in type I patients, and 4.5 ± 1.8 cm (range 1.7–9.4 cm) and 55.2 ± 53.7 cm3 (range 1.3–200.9 cm3) in type II patients, respectively. Based on the MRI T2 signal intensity, 9 (33.3%) fibroids were classified as intermediate intensity, 18 (66.7%) as low intensity in the type I group, 11 (39.3%) were classified as intermediate intensity, and 17 (60.7%) as low intensity in the type II group. In this study, 74.1%, 33.3% and 22.2% of the patients with type I submucosal fibroids complained of menorrhagia, dysmenorrhoea and anaemia, compared to 67.9%, 42.9% and 32.1% in patients with type II submucosal fibroids. No significant difference in baseline characteristics between the two groups was observed (p > 0.05).
Table 1. Comparison of demographic characteristics between patients with type I and type II submucosal fibroids.
HIFU treatment evaluation during and after treatment
As shown in , no significant difference was observed between the two groups in HIFU treatment setting and procedure (p > 0.05), including sonication power, sonication time, sonication time/h, and total energy used. With the utilisation of similar sonication power, sonication time, and total energy to ablate the fibroids, a non-perfused volume (NPV) ratio of 83.0 ± 17.3% was achieved in the type I group; the NPV ratio of fibroids was 92.0 ± 9.5% in the type II group. The NPV ratio of fibroids was significantly higher in patients with type II submucosal fibroids than in patients with type I.
Table 2. Comparison of HIFU treatment results between patients with type I and type II submucosal fibroids.
Fibroid-related symptom improvement and fibroid volume changes after HIFU treatment
All the patients completed 1 month, 6 months, and 12 months follow-up. The fibroid-related symptoms were relieved after HIFU treatment. Menorrhagia is the most common symptom in these patients and was seen in 74.1% of the patients in group type I and 67.9% of the patients in group type II; At 12 months after HIFU treatment, only 18.2% of patients in group type I and 4.8% of patients in group type I still complained of menorrhagia. For the patients with anaemia, all of them reported the level of haemoglobin returned to normal at the 12-month follow-up. There were no significant differences in improvement of fibroid-related symptoms between the two groups (p > 0.05).
shows the changes in fibroid volume: 1 month after HIFU treatment the fibroid volumes in group type I and type II were 23.7 ± 43.7 cm3 and 31.1 ± 33.8 cm3, respectively; 6 months and 12 months after HIFU treatment the volume of the fibroids further decreased in both groups (). During the follow-up period, the submucosal fibroids completely disappeared in five patients in the type I group and in six patients in the type II group.
Figure 2. MR images (T2WI) obtained from a 37-year-old patient with submucosal fibroids. (A) Pre-procedure T2WI shows a 5.5 × 3.8 × 4.3 cm submucosal fibroid located at the fundus of the uterus (arrow). (B) T2WI obtained 1 month after HIFU shows the size of the fibroid was 3.0 × 2.0 × 2.3 cm (arrow). (C) T2WI obtained 6 months after HIFU shows the size of the fibroid was 0.5 × 0.4 × 0.4 cm (arrow). (D) T2WI obtained 12 months after HIFU shows the treated fibroid tumour has disappeared (arrow).
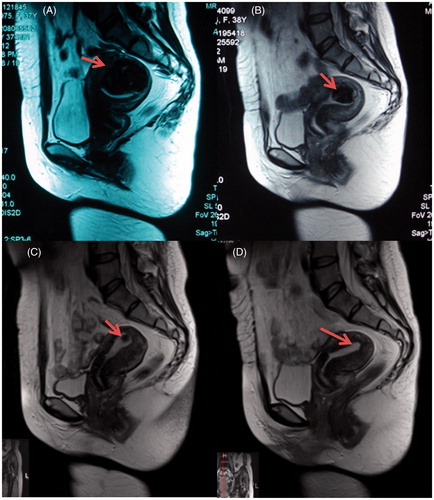
Table 3. Comparison of fibroid volume changes between patients with type I and type II submucosal fibroids after HIFU treatment.
HIFU treatment safety evaluation during and after treatment
HIFU treatment was performed under conscious sedation so that patients could report any discomfort during the procedure. In this study the most common adverse events during HIFU ablation were sciatic or buttock pain, lower abnormal pain and discomfort on skin. These pains were often transient and mild. shows that no significant difference was observed between the two groups in the above-mentioned adverse effects (p > 0.05).
Table 4. Incidence rates of adverse effects during the HIFU procedure.
shows the post-HIFU adverse effects and complications. After HIFU treatment a few patients (3.7%) in the type I group complained of leg pain; 11.1% of the patients in the type I group and 7.1% of the patients in the type II group reported sciatic/buttock pain; 29.6% of the patients in the type I group and 28.6% of the patients from the type II group complained of lower abdominal pain. However, the pain score was below 4 points and these pains subsided within 5 days. In this study, the most common post-HIFU adverse effect was vaginal discharge or bleeding, which was seen in 29.6% of the patients in the type I group and 32.1% of the patients in the type II group. Vaginal discharge or bleeding subsided within 1 week in most of the patients. However, because of the close anatomical relationship between the submucosal fibroid and the endometrium, it is possible for a part of the necrotic tissue of the treated submucosal fibroids or the whole tumor to be discharged from the vagina. In this study, vaginal expulsion was observed in six patients from each group. In addition, two patients complained of pain of uterine contraction because of the necrotic fibroid tissue obstructing their cervixes. An intravaginal forceps-assisted procedure was performed to help remove the obstructed necrotic fibroid tissue. No significant difference was observed between the two groups in the rates of adverse effects and complications (p > 0.05).
Table 5. Comparison of adverse effect incidence rates and complications between patients with type I and type II submucosal fibroids after HIFU treatment.
Discussion
Submucosal fibroids often cause heavy menstrual bleeding and anaemia [Citation1]. The purpose of the treatment is to relieve the symptoms. As a minimally invasive technique, hysteroscopic resection was first introduced by Neuwirth to treat submucosal fibroids in the 1970s [Citation19]. However, complete removal of submucosal fibroids remains a challenge. Several studies showed that complete removal by hysteroscopic myomectomy was only achieved in type 0 submucosal fibroids [Citation6,Citation7]. The present study showed that both type I and type II submucosal fibroids could be satisfactorily ablated in one HIFU session with an average operating room time of less than 90 min. Although Stewart et al. reported that achieving a small NPV ratio with MRgFUS provided sustained symptom relief, attaining a large NPV ratio is the goal of thermal ablative treatment because many studies have shown that the NPV ratio is related to long-term symptom relief and reintervention rate [Citation20,Citation21]. In this study, the NPV ratio of 83.0 ± 17.3% was achieved in type I patients and the NPV ratio of 92.0 ± 9.5% was achieved in type II patients (). One-year follow-up results showed that the fibroid-related symptoms were relieved and the fibroid volume reduced significantly (). In comparison with previous studies, the shrinkage rate in this study is much larger [Citation17,Citation18]. The large reduction rate could be explained by the large NPV ratio and the spontaneous vaginal expulsion. No patient had other reintervention procedures during the 1-year follow-up period. Therefore HIFU seems to be comparable to hysteroscopic myomectomy in treating type I and type I submucosal fibroids. We further compared the therapeutic results of the two groups. The interesting finding was that by using similar sonication power, sonication time, and acoustic energy the NPV ratio achieved in the type II group of patients was significantly higher than that of type I patients (p < 0.05). This phenomenon might be explained by the type II submucosal fibroids having higher intramural extension than those of type I. The small blood vessels were enriched in basilar membrane; with less endometrium covering the inner surface of the fibroids there were fewer blood vessels. Therefore, it is likely that it was easier to deposit the acoustic energy at the target of type II submucosal fibroids than for type I because the blood perfusion around type II was less than that for type I.
Another important concern for the management of uterine fibroids is safety. Hysteroscopic myomectomy is associated with complications including infection, bleeding, uterine perforation, visceral injury, hyponatraemia, pulmonary oedema, heart failure, cerebral oedema, hypoxia, and in rare cases may cause death [Citation22,Citation23]. In addition, high post-operative intrauterine synechia rates of 7.5–45% have been reported [Citation22–25]. As a non-invasive technique, HIFU may lead to protein denaturation and irreversible cell death through selective coagulative necrosis by raising local temperatures to above 60 °C in a very well-defined volume without damaging surrounding tissues [Citation26]. Recently, Wang et al. reported that HIFU ablation could be safely used to ablate submucosal fibroids without damaging the endometrium [Citation18]. In comparison with hysteroscopic myomectomy, HIFU is a non-invasive treatment modality and distension of the uterus was not needed during the procedure; thus, the potential complications associated with fluid overload in the procedure of hysteroscopic myomectomy were not observed in the present study. Another minimally invasive treatment, UAE is related to a higher rate of post-procedural complications including vaginal discharge, post-puncture groin haematoma, and serious inflammatory complications or sepsis, as well as post-embolisation syndrome (pain, fever, nausea, vomiting) [Citation27–29]. HIFU is not associated with most of the above-mentioned complications. In this study, HIFU was performed under conscious sedation. Patients reported transient leg pain, sciatic/buttock pain, lower abdominal pain, and ‘hot’ skin sensation during HIFU sonication (). However, these adverse effects were transient and the pain score was less than 4 points. Immediately after HIFU treatment we observed some common adverse effects including lower abdominal pain, sciatic/buttock pain, and vaginal discharge or bleeding. Based on the Society of Interventional Radiology (SIR) classification system for complications by outcome, these adverse effects were mild and the pains often subsided within 5 days; most of the vaginal discharge or bleeding subsided in 1 week. A few cases in the group of type I patients reported leg pain after HIFU treatment because of temporary sacral nerve irritation. The leg pain lasted for 2 weeks to 2 months and thus was defined as a major adverse effect. We also observed 12 cases from both groups that reported spontaneous vaginal expulsion and two cases complained of cramps because the necrotic fibroid tissue obstructed the cervix. The symptoms disappeared after the obstructed necrotic fibroid tissue was removed by intravaginal forceps-assisted procedure. As the fibroid tumour can be removed completely without using invasive procedures, spontaneous vaginal expulsion could be counted as an advantage of HIFU. During the follow-up period, no patient reported other adverse effects such as infection, bowel injury, or nerve injury. Our results did not show any significant difference between the two groups in the rate of the adverse effects and complications (). Therefore, HIFU can be considered a safe treatment in the management of submucosal fibroids.
This study is limited because of the small number of subjects. It is also limited by the relatively short follow-up time. In addition, we did not check the uterus with hysteroscopy after HIFU treatment, so we do not know if intrauterine synechiae occurred. Future studies with a large number of subjects and longer follow-up time are needed to figure out the long-term recurrence rate and the reintervention rate, as well as the pregnancy outcomes after HIFU treatment.
Conclusions
In summary, the present study demonstrated that HIFU can be used safely to treat submucosal fibroids effectively. Based on our small sample study size, HIFU is suitable for both type I and type II submucosal fibroids. It seems that type IIsubmucosal fibroids are more sensitive to HIFU ablation. Future studies with larger sample sizes and longer follow-up times to investigate the long-term results, including long-term symptom relief, pregnancy outcomes, and recurrence rate, as well as the reintervention rate are needed.
Declaration of interest
Lian Zhang is a senior consultant of the Chongqing Haifu company. The other authors have no conflicts of interest to declare. The authors alone are responsible for the content and writing of the paper.
References
- Puri K, Famuyide AO, Erwin PJ, Stewart EA, Laughlin-Tommaso SK. Submucosal fibroids and the relation to heavy menstrual bleeding and anemia. Am J Obstet Gynecol 2014;210:38. e1–7
- Chabbert-Buffet N, Esber N, Bouchard P. Fibroid growth and medical options for treatment. Fertil Steril 2014;102:630–9
- Wamsteker K, Emanuel MH, de Kruif JH. Transcervical hysteroscopic resection of submucosal fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol 1993;82:736–40
- Muñoz JL, Jiménez JS, Hernández C, Vaquero G, Pérez Sagaseta C, Noguero R, et al Hysteroscopic myomectomy: our experience and review. JSLS 2003;7:39–48
- Campo S, Campo V, Gambadauro P. Short-term and long-term results of resectoscopic myomectomy with and without pretreatment with GnRH analogs in premenopausal women. Acta Obstet Gynecol Scand 2005;84:756–60
- Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol 1999;93:743–8
- Hart R, Molnar BG, Magos A. Long term follow up of hysteroscopic myomectomy assessed by survival analysis. Br J Obstet Gynaecol 1999;106:700–5
- Homer H, Saridogan E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil Steril 2010;94:324–30
- Moss JG, Cooper KG, Khaund A, Murray LS, Murray GD, Wu O, et al Randomized comparison of uterine artery embolization (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG 2011;118:936–44
- Zhang L, Chen WZ, Liu YJ, Hu X, Zhou K, Chen L, et al Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol 2010;73:396–403
- Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. J Cancer Res Ther 2010;6:414–20
- Stewart EA, Gedroyc WM, Tempany CM, Quade BJ, Inbar Y, Ehrenstein T, et al Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol 2003;189:48–54
- Quinn SD, Gedroyc WM. Thermal ablative treatment of uterine fibroids. Int J Hyperthermia 2015. Mar 27:1–8
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 2009;34:584–9
- Zhao WP, Chen JY, Zhang L, Li Q, Qin J, Peng S, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2-weighted MR imaging. Eur J Radiol 2013;82:e43–9
- ter Haar G. High intensity ultrasound. Semin Laparosc Surg 2001;8:77–89
- Jung SG, Yoon SW, Park H, Lee C. Potential exploratory use of MR-guided focused ultrasound for disconnection of symptomatic intracavitary submucosal uterine myoma. J Vasc Interv Radiol 2011;22:1635–7
- Wang W, Wang Y, Wang T, Wang J,Wang L,Tang J. Safety and efficacy of US-guided high-intensity focused ultrasound for treatment of submucosal fibroids. Eur Radiol 2012;22:2553–8
- Neuwirth RS, Amin HK. Excision of submucus fibroids with hysteroscopic control. Am J Obstet Gynecol 1976;126:95–9
- Stewart EA, Rabinovici J, Tempany CM, Inbar Y, Regan L, Gostout B, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 2006;85:22–9 . Erratum. Fertil Steril 2006;85:1072
- Quinn SD, Vedelago J, Gedroyc W, Regan L. Safety and five-year re-intervention following magnetic resonance-guided focused ultrasound (MRgFUS) for uterine fibroids. Eur J Obstet Gynecol Reprod Biol 2014;182:247–51
- Paul D. Indman. Hysteroscopic treatment of submucosal myomas. Clin Obstet Gynecol 2006;49:811–20
- Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol 2002;14:409–15
- Touboul C, Fernandez H, Deffieux X, Berry R, Frydman R, Gervaise A. Uterine synechiae after bipolar hysteroscopic resection of submucosal myomas in patients with infertility. Fertil Steril 2009;92:1690–3
- Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucosal fibroids. Curr Opin Obstet Gynecol 2013;25:332–8
- Zhang L, Zhang W, Orsi F, Chen W, Wang Z. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia 2015. Jan 22:1–5
- Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG 2002;109:1262–72
- Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2012;5:CD005073
- Vashisht A, Studd JW, Carey AH, Burn P. Fatal septicaemia after fibroid embolisation. Lancet 1999;354(9175):307–8
