Abstract
Purpose: This study examines the effect of passive hyperthermia on interhemispheric resting state functional connectivity and the correlation between interhemispheric resting state functional connectivity and efficiency of a succedent working memory task. Materials and methods: We performed voxel-mirrored homotopic connectivity (VMHC) analyses on resting state MRI data and a one-back task from 14 healthy subjects in both HT (hyperthermia, 50 °C) conditions and normal control (NC, 25 °C) conditions. The group analyses of the differences for VMHC between the two conditions and the correlation analysis between the VMHC and the reaction time (RT) of the one-back task were performed with the statistical parametric mapping software package and the software REST. Results: Compared with NC conditions, HT conditions increased VMHC in the cuneus, the postcentral gyrus, and the fusiform gyrus. No region showed decreased VMHC in the HT group in comparison with the NC group. For NC conditions, negative correlations were demonstrated between RT of the one-back task and VMHC in bilateral superior temporal gyrus, and bilateral middle frontal gyrus; for HT conditions, negative correlations were demonstrated between RT and VMHC in bilateral inferior frontal gyrus, bilateral middle frontal gyrus, as well as cerebellum posterior lobe. Conclusion: Passive heat stress can impact the interhemispheric information interactions at resting state and the VMHC deficits may play an important role in cognitive dysfunction.
Introduction
As a very common risk factor in many work places (e.g. manufacturing workshops, coal mines, military operations, fire-fighting and outdoor sports), passive heat exposure can alter the cognitive function of the brain. Although passive hyperthermia can induce the alterations of many cognitions, such as working memory [Citation1–3], attention [Citation4–6], vigilance [Citation7], recognition [Citation8] and tracking performance [Citation3], the pathophysiological substrate or brain activity related to them remains a relatively unexplored area.
Interhemispheric communication is critical to understand the pathophysiological substrate of cognitive function and may be a particularly fruitful approach to understand the lateralisation of function for cognitive processing [Citation9]. For although the hemispheres differ in the manner in which they process various kinds of cognitive and emotional information [Citation9–11], interhemispheric interaction leads to enhanced performance by distributing the processing load across the hemispheres [Citation12], and when task requirements are demanding, interhemispheric interaction is increased [Citation9]. Considering that under heat stress cognitive tasks become more difficult than in a normal comfortable environment, we presumed that heat stress would increase the interhemispheric interaction.
Recently, advanced brain imaging techniques have increased the capabilities for visualising brain activities, and also have been utilised to research the pathophysiological substrate for the alteration of cognition during passive hyperthermia. Hocking et al. [Citation13] reported an increase of amplitude and a decrease of latency in the frontal and occipitoparietal regions under heat stress by steady-state, visually evoked potentials (SSVEPs). By recording the mismatch negativity (MMN) component of event-related potentials (ERPs), Sun et al. [Citation8] suggested that the MMN component could be an appropriate index to assess cognitive function under heat stress. With favourable spatial resolution, functional magnetic resonance imaging (fMRI) was also adopted in the study of cognitive function under heat stress. With fMRI, we have revealed that passive hyperthermia enhanced the activity in the right superior frontal gyrus and depressed the activity in the right middle occipital gyrus, left inferior parietal lobule and left culmen in the alerting network, enhanced the activity in the temporal lobe and depressed the activity in the frontal lobe, parietal lobe and occipital lobe in the orienting network, enhanced the activity in the dorsolateral prefrontal cortex (DLPFC) of the executive network [Citation5]. We also found passive hyperthermia decreased the small-world property of the resting state network [Citation14], decreased functional connectivity involved with the medial orbitofrontal cortex, temporal lobe and occipital lobe, and increased functional connectivity mainly located within the limbic system of the resting state network [Citation15]. However, little is known about the alterations in the functional interaction between the cerebral hemispheres, although interhemispheric communication is very important to understanding brain function [Citation9]. So the main purpose of this study was to observe interhemispheric communication during passive hyperthermia.
Resting state fMRI (rsfMRI) which is widely used to investigate brain networks that exhibit correlated fluctuations is a technique that permits assessment of inter- as well as intrahemispheric functional connectivity (FC) [Citation16]. Homotopic functional connectivity is a validated method to integrate brain function underlying coherent cognition, behaviour and consciousness [Citation17,Citation18]. Recently, voxel-mirrored homotopic connectivity (VMHC), a feasible method to evaluate the resting state functional connectivity (RSFC) quantitatity between each voxel in one hemisphere and its mirrored counterpart in the opposite hemisphere, has been developed [Citation19]. Different from regional homogeneity that measures specifically the synchronisation of regional resting-state fluctuation activity among neighbouring voxels within a local region, VMHC is defined as the high degree of synchrony in spontaneous activity between geometrically corresponding interhemispheric regions. And it has been widely used in many diseases such as schizophrenia [Citation20], cocaine addiction [Citation21], depressive disorder [Citation22,Citation23], dyspepsia [Citation24] and migraine patients [Citation25], all of which suggest that the method is sensitive to abnormal interhemispheric FC in psychopathology. To our knowledge, no studies on the interhemispheric RSFC changes during passive hyperthermia have been investigated, which restrict the full understanding for the impairment of passive hyperthermia on cognition.
In this study, then, we aimed to observe the interhemispheric communication during passive hyperthermia with VMHC, and assess the relation between the interhemispheric communication and cognitive function. Considering that VMHC changes have been found in many diseases, and when task requirements are demanding, interhemispheric interaction is increased [Citation9], we predicted that passive heat stress can increase VMHC in resting state and increase the correlation between VMHC and cognitive tasks.
Materials and methods
Participants
Fifteen healthy right-handed male college students (22.5 ± 1.75 years, ranging from 19–25 years) were recruited from the campus of a local university through posters. All of the participants had no history of neurological or psychiatric disorders and had never participated in any fMRI experiments before. Exclusion criteria were abnormal visual acuity, neurological or psychiatric illness, drug or alcohol abuse, sensitivity to heat stress, or contraindication to MRI. In addition, the subjects could withdraw during the course of the experiment if they could not tolerate the heat. Informed consent forms and completed comprehensive medical questionnaires were obtained from all the participants before the experiment. The research protocol was approved by the ethical committee of our hospital.
Procedure
Using a counter-balanced design, subjects then completed two experimental trials in hyperthermia (HT) conditions with a 40-min exposure at 50 °C and 40% relative humidity (rH) and normal control (NC) conditions with a 40-min exposure to a temperature of 25 °C and 40% rH, separated by at least 7 days of recovery. All participants under both conditions wore a thermal lab-suit covering the whole body including the head. The suit was designed with a soft embedded pipeline in which the hot water would circulate to simulate environmental heat during fMRI scanning. After the 40-min heat exposure the participants entered the MRI room for scanning. During the scanning with water circulating throughout the pipeline, the temperature of the water was controlled at 40 °C in HT conditions and at 28 °C in NC conditions with a subsidiary water temperature control device (OWK-C, Germany Ouchida Int’l Group). To optimally simulate the environment, participants wore a respiration hood in which the hot air circulated with the air temperature controlled by the same device. During the resting-state scan, the participants were instructed to lie quietly and remain still with their eyes open, and not think of anything. The resting-state fMRI scans took about 20 min. During the test sessions, the participants were instructed to drink water ad libitum to ensure body weight changed less than 1%.
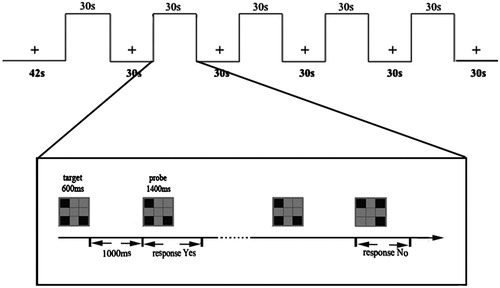
Given that passive hyperthermia impaired working memory [Citation1–3], which is crucial for many other cognitions such as perception [Citation26], attention [Citation27–29], and visual search [Citation30], after MRI scanning we took the participants back to the environmental chamber and performed a visual-spatial working memory task to estimate behavioural effects of cognition under different heat stresses. In the working memory task the stimuli were presented in separate blocks with a modified one-back paradigm. All stimulus pictures were presented with three spatial units lighting pseudo-randomly in the rectangular area which was composed of an array of spatial positions: 3 × 3 (horizontal/vertical viewing 4 °). Each trial began with target stimuli (600 ms), and probe stimuli for 1400 ms alternately, with an inter-stimulus interval (ITI) of 1000 ms. Participants responded ‘yes’ or ‘no’ to indicate whether the probe was identical to the target, followed by an 800-ms blank delay period. It was designed so that 50% of the probe pictures were identical to the target pictures. One run contained 60 trials and each participant was tested for 1 run. Each block lasted 30 s and consisted of 10 pairs of stimuli per trial (see ).
Figure 1. Stimulus pattern of the working memory task for fMRI block design. There was a 12 s dummy scan ahead of the experiment. At the first 30 of the run, the participants should fix on the cross point and do nothing, followed by stimulus images. Participants were asked to judge whether the probe matched the target in the sample display with the whole process being limited to 30 s. Then another fixation followed was continued 30 s. The procedure mentioned above went on alternately for five blocks with a dummy scan of 30 s as the ending. The whole experiment costs 5 min 42 s.
In addition, the rectal temperature and net weight of each participant were recorded before and after the experiments using a rectal temperature logger (ZWJ-2, Honour Instruments, Tianjin, China) and an electronic weightometer (Xiangshan, EF901, China) respectively under both conditions.
Data acquisition
The resting-state scans were obtained for all of the participants using a GE Signa 3.0 T scanner (General Electric, Milwaukee, Wisconsin). The participants lay in a supine position with their heads fixed in place by foam pads to minimise head translation and rotation movements. Each scan consisted of 200 EPI functional volumes with the following parameters: TR 2000 ms, TE 40 ms, flip angle (FA) 90°, number of slices 29, matrix 64 × 64, field of view (FOV) 24 × 24 cm2, thickness/gap 4/0 mm, acquisition voxel size 3.75 × 3.75 × 4 mm3. Additionally, a high resolution T1-weighted sequence was obtained: 115 slices, TR 11.1 ms, TE 4.9 ms, slice thickness 1.4 mm, FOV 24 × 24 cm2, FA 20°.
Data preprocess analysis
Data preprocessing was conducted in Matlab (Mathworks) using the statistical parametric mapping software package (SPM8, http://www.fil.ion.ucl.ac.uk/spm). Data of individuals that met the following criteria were discarded: head motion more than 1 mm or rotation more than l.5°. Then the images were normalised and resampled to 3 × 3 × 3 mm3. The images generated were processed using spatial smoothing with a 6 mm full-width-at-half-maximum Gaussian kernel, temporal band-pass filtering (0.01–0.08 Hz) and linearly detrended removal. Several sources of spurious covariates along with their temporal derivatives were then removed from the data by using linear regression, including six head motion parameters obtained by rigid body correction, the signal from a ventricular region of interest (ROI), and the signal from a region centred in the white matter [Citation31]. Finally, the residual time series for each participant was registered to a symmetric Montreal Neurological Institute (MNI) template and was utilised to calculate the homotopic resting state FC.
Interhemispheric correlation
The VMHC computation was performed with REST software [Citation32]. For each participant, the homotopic resting-state FC was computed as the Pearson correlation coefficient between each voxel’s residual time series and that of its mirrored interhemispheric counterpart. Correlation values were then Fisher z-transformed to improve the normality. The resultant values generated the VMHC maps and were applied for group comparisons.
Statistical analysis
All statistical analyses of body weight, temperature and behavioural data of working memory task were performed using SPSS, version 17.0. The statistical significance was accepted at p < 0.05. A two-way within-subject analysis of variance (ANOVA) for repeated measures (time vs. group) was used to assess the main effects of the group (control or hyperthermia) and time (before or after the experiment) with regard to changes in Tcore or body weight. Univariate post-hoc tests with Tukey corrections for multiple comparisons were run to investigate the interaction of time and group. In addition, an ANOVA for repeated measures (group) was used to assess the hyperthermia effect on working memory.
Individual-level VMHC maps were entered into a group-level voxel-wise two-tailed paired t-test. The resulting statistical map was set at p < 0.005 for multiple comparisons using Gaussian random field (GRF) theory (cluster significance: p < 0.005, AlphaSim corrected).
In addition, we further assessed the relationships between VMHC and RT for the working memory task in a voxel-wise manner for NC conditions and HT conditions respectively. The threshold was set at a corrected p < 0.01, estimated using the same parameters as the group comparison analysis of VMHC.
Results
Body weight and body temperature
No participant for whom body weight changed more than 1% during the experiment was found, and the group analyses showed that there were no significant effects of thermal conditions and time (pre- or post-experiment) on body weight (F(1, 28) = 1.808, p = 0.19 > 0.05).
For body temperature, repeated measures ANOVA showed that the interaction between groups and time was significant: F(1, 28) = 79.745, p < 0.001. Whereas body temperature did not differ between the two thermal conditions pre-experiment, F(1, 28) = 1.713, p = 0.201 > 0.05, HT conditions resulted in significantly higher body temperature than NC conditions post-experiment: F(1, 28) = 63.398, p < 0.001. These data demonstrate the efficacy of passive hyperthermia in this study.
One participant was removed from further analysis due to extensive head motion (more than 1 mm).
VMHC: group differences
and show the group comparisons of VMHC values between the two thermal conditions. Compared with NC conditions, HT conditions increased VMHC in the cuneus, the postcentral gyrus, and the fusiform gyrus. No region showed decreased VMHC in the HT group compared to the NC group.
Figure 2. Regions showing group differences in voxel-mirrored homotopic connectivity. Warm colors indicate increased VMHC in hyperthermia condition (50 °) compared to normal control condition (25 °). Group differences were a corrected cluster threshold of p = 0.005. The Z-score bar is shown at the middle of the figure.
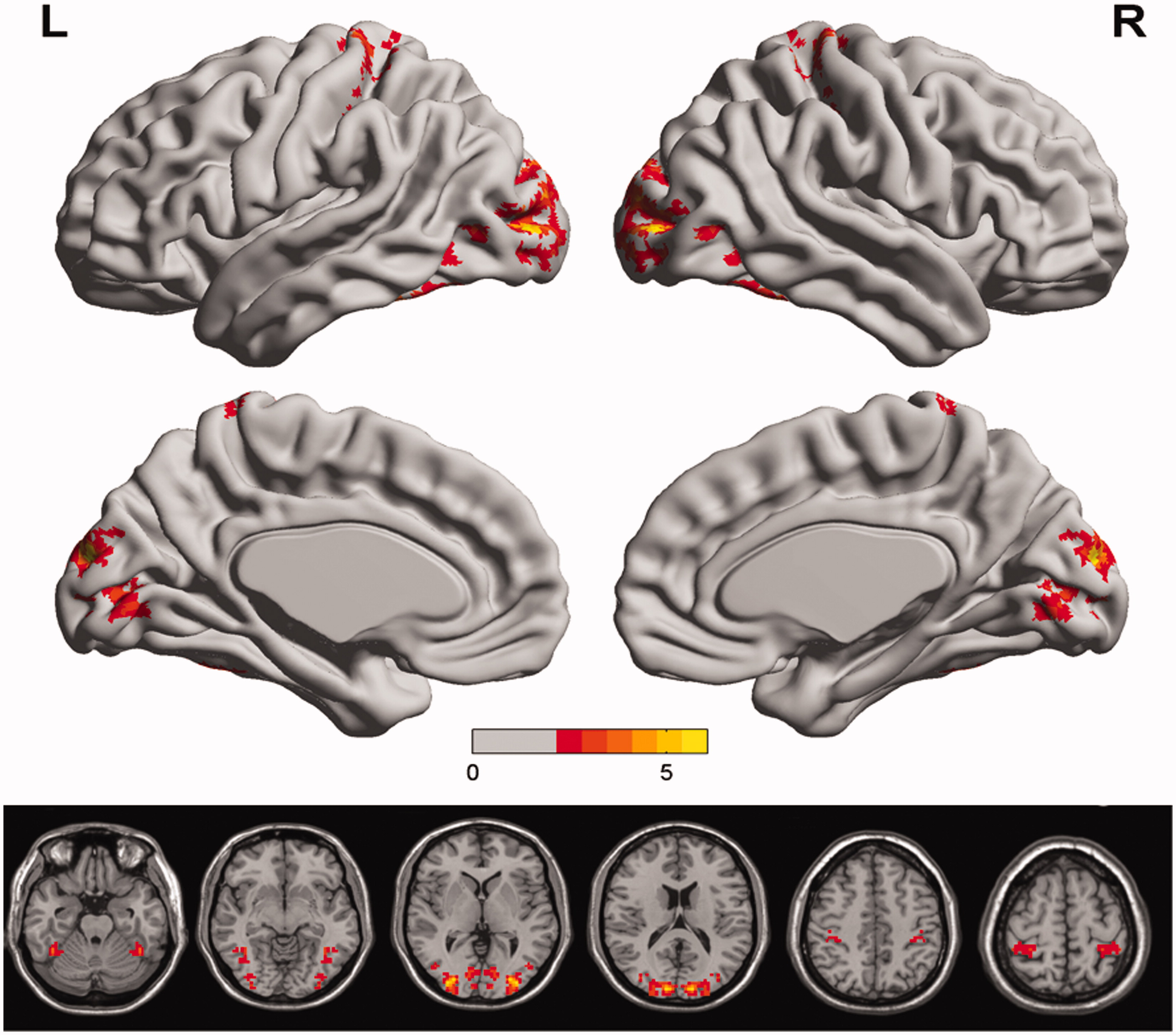
Table 1. Regions showing group differences in voxel-mirrored homotopic connectivity.
Working memory task results and correlations between VMHC and RT
Similar to our previous study, compared to NC conditions, HT conditions increased RT of working memory significantly, F(1, 13) = 136.352, p < 0.001, while HT conditions did not change the accuracy of working memory, F(1, 13) = 0.136, p = 0.719. The details for RT and accuracy of working memory are showed in .
Table 2. Mean RT (and SD) and accuracy (and SD) for each condition.
Further analyses showed some significant correlations between VMHC and RT for working memory for both NC conditions and HT conditions. For NC conditions, negative correlations were demonstrated between RT of working memory and VMHC in bilateral superior temporal gyrus, and bilateral middle frontal gyrus (, and ); for HT conditions, negative correlations were demonstrated between RT of working memory and VMHC in bilateral inferior frontal gyrus, bilateral middle frontal gyrus, as well as cerebellum posterior lobe (, , and ).
Figure 3. Regions showing significant negative correlations between VMHC and reaction time of working memory in normal control enviroment. The region in bilateral superior temporal gyrus, and bilateral middle frontal gyrus showing significant positive correlation between the VMHC and reaction time of working memory task; cool colors indicate negative correlations. The statistical threshold was p < 0.005. (The correction threshold was determined by a Monte Carlo simulation). The Z-score bars are shown at the middle of the figure.
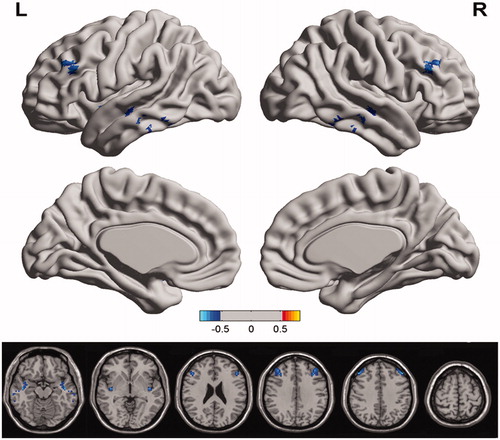
Figure 4. The scatterplots of the VMHC values in normal control enviroment along the x-axis, and WM score along the y-axis, with A for bilateral superior temporal gyrus and B for bilateral middle frontal gyrus.
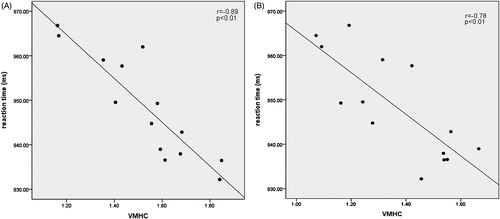
Figure 5. Regions showing significant negative correlations VMHC and reaction time of working memory under heat stress. The region in bilateral inferior frontal gyrus, bilateral middle frontal gyrus, as well as cerebellum posterior lobe showing significant negative correlation between the VMHC and reaction time of working memory task; cool colors indicate negative correlations. The statistical threshold was p < 0.005. (The correction threshold was determined by a Monte Carlo simulation). The Z-score bars are shown at the middle of the figure.
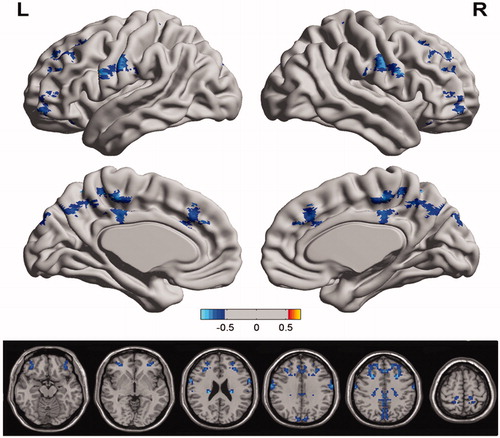
Figure 6. The scatterplots of the VMHC values in hyperthermia enviroment along the x-axis, and WM score along the y-axis, with A for bilateral cerebellum posterior lobe, B for bilateral middle frontal gyrus, and C for bilateral inferior frontal gyrus.
Table 3. Regions showing significant negative correlations between VMHC and RT of working memroy.
Discussion
Here, VMHC was applied for the first time to investigate interhemispheric RSFC of human brain under heat stress. Significant increases in VMHC were found under heat stress in the cuneus, the postcentral gyrus, and the fusiform gyrus. No areas showed decreased VMHC under heat stress. Further analysis revealed significant negative correlations between VMHC in bilateral superior temporal gyrus, bilateral middle frontal gyrus and RT of working memory task under NC conditions, between VMHC in bilateral inferior frontal gyrus, bilateral middle frontal gyrus, as well as cerebellum posterior lobe and RT of working memory task under heat stress. These VMHC variations may play an important role in the cognitive deficits of people under heat stress.
VMHC of resting state
Although no study has yet researched interhemispheric functional interactions of resting state under heat stress, altered functional interactions and brain activities have been observed with our previous studies [Citation14,Citation15]. The results of these studies also showed that altered functional interactions are not entirely symmetrical in the left and right cerebral hemispheres, suggesting that heat stress probably impaired interhemispheric information interactions. The current study indicated that heat stress increased VMHC of resting state in the cuneus, the postcentral gyrus, and the fusiform gyrus, which support the view of network reconfiguration of the brain in response to changing environmental demands [Citation33]. In addition, there were only increased VMHC and no decreased VMHC, which are in accordance with the view that when task requirements are demanding, performance is enhanced by distributing processing across the hemispheres [Citation9], for heat stress can increase the difficulty of cognitive tasks. Moreover, considering that passive hyperthermia decreased clustering coefficient of the brain network [Citation14], the current results maybe evidence of the conclusion that greater cognitive effort causes emergence of a more long-distance synchronisation between brain regions and increases the global efficiency of the network for information transfer [Citation33].
Disrupted VMHCs in the fusiform gyrus have been discovered in patients [Citation23]. Fusiform gyrus is a functional brain area in the visual recognition network [Citation34]. Therefore, we speculate that the increased information communication of bilateral fusiform gyrus should be an important mechanism in the impairment of face recognition efficiency under heat stress, as this deficit has been confirmed by our previous study [Citation8].
Cuneus is classically related to visual information processing [Citation35], and seems to be to integrate the somatosensory information with other sensory stimuli and cognitive processes such as attention, learning and memory [Citation36]. In this study, increased VMHC in the cuneus may contribute to thermal perception. As heat stress can impair attention and memory [Citation1,Citation4–6], the current results indicate that aberrant interhemispheric functional connectivity in cuneus should be an important mechanism of the impairments.
The postcentral gyrus is the primary somatosensory region. The increased VMHC under heat stress probably reflects the thermal sensation of the body, as a recent animal study has revealed that stress increases connectivity within the somatosensory networks [Citation37]. In addition, the primary somatosensory cortex activity correlates with sensory perception and task performance [Citation38,Citation39]. As we did not measure sensory perception and task performance in the current study, the correlation between VMHC of the primary somatosensory cortex activity and sensory perception or task performance under heat stress needs to be explored in future studies.
VMHC correlated with working memory
In order to indicate the relationship between VMHC of resting state and behavioural performance of succedent working memory under heat stress, we first analysed the relationship between them under NC conditions. To the best of our knowledge, this is also the first observation of VMHC correlated with working memory. The results showed that the capacity of succedent working memory elevated as the VMHC in the bilateral superior temporal gyrus, and bilateral middle frontal gyrus increased. As an essential cognitive function, working memory is defined as the system for the temporary maintenance and manipulation of information, necessary for the performance of such complex cognitive activities as comprehension, learning, and reasoning [Citation40]. DLPFC, parietal lobe, and temporal lobe are important functional areas of working memory [Citation41–44]. The current results confirmed the importance of DLPFC and temporal lobe for working memory one more time. In addition, it also suggests that VMHC in the bilateral superior temporal gyrus, and bilateral middle frontal gyrus should be another neuromechanism for working memory.
Furthermore, the results revealed a significant negative correlation between VMHC in the bilateral inferior frontal gyrus, bilateral middle frontal gyrus, as well as cerebellum posterior lobe and RT of succedent working memory task under heat stress. Compared to NC conditions, a negative correlation between VMHC in the bilateral inferior frontal gyrus, cerebellum posterior lobe and RT of working memory task appeared, while the negative correlation between VMHC in bilateral superior temporal gyrus and RT disappeared. In addition, the number of voxels in the bilateral middle frontal gyrus for which VMHC was correlated with RT in HT condition was larger than in NC conditions. So the results of this study proved that when task requirements were demanding, performance was enhanced by distributing processing across the hemispheres [Citation9], and greater cognitive effort caused emergence of a more long-distance synchronisation between brain regions and increased the global efficiency of the network for information transfer [Citation33] for another time.
The DLPFC, including the middle frontal gyrus, inferior frontal gyrus and other brain areas, is crucial to the functional cortex for many superior cognitive performances. And previous studies have proved that heat stress can affect the function of DLPFC [Citation1,Citation5,Citation45]. Now, the current results indicate that the brain completes working memory tasks better if more information interacts between bilateral DLPFC under heat stress. The result which surprised us most was the significant negative correlation between the VMHC of bilateral cerebellum posterior lobe and the RT of working memory. Although the cerebellum is mainly responsible for body balance, increased cerebral metabolic rate and cerebral blood flows in the cerebellum have been observed [Citation45,Citation46]. In fact, the cerebellum receives sensory information input from all parts of the body, and has been viewed as being involved in the planning and initiation of movement [Citation47,Citation48]. Thus, the correlation between the VMHC of bilateral cerebellum and the RT of working memory might be related to the thermal sensation and behavioural thermoregulation mechanism of motor planning, intention and control to overcome the effect of heat stress on working memory. In sum, these results suggest that under heat stress the brain conducted a different mode of VMHC to complete the working memory task. That is to say, the brain recruits new regions not typically utilised in working memory operations to compensate for the stress imposed by hyperthermia, which has been confirmed by other studies [Citation1,Citation5].
For the impairments of passive hyperthermia on working memory that have been observed [Citation1,Citation3], our results indicate that the change of VMHC may be one of the neuromechanisms for these impairments. Our previous event-related blood-oxygen-level dependent (BOLD)-fMRI study revealed that performing working memory tasks enhanced the activity strength of bilateral DLPFC and right intraparietal sulcus [Citation1]. The results of the current study have extended the view that passive heat stress affects not only brain activity of local brain cortex but also the interhemispheric information interactions. In addition, considering that it has been shown that resting-state BOLD activity has predicted performance in other modalities, such as in motor [Citation49] and visual tasks [Citation50], it is even conceivable that interhemispheric RSFC under heat stress could serve as a biomarker to predict change in cognitive performance such as working memory. This deduction needs to be confirmed in future studies for other cognitive functions.
We acknowledge that there are several limitations in the current study. First, the group’s size was relatively small; a larger sample study further researching interhemispheric information interactions in passive hyperthermia conditions is needed in the future. Second, there are existing asymmetries in the cortical structure of the human brain. However, we tried to mitigate these asymmetries with a symmetric template and by smoothing the functional data. Third, although the VMHC deficits might be associated with white matter integrity and local grey matter volume abnormalities [Citation10,Citation51–53] no voxel-based morphometry or white matter diffusivity was analysed in these VMHC altered regions. Fourth, additional neuropsychological tests are also needed, and we will investigate the associations between cognitive dysfunction and VMHC deficits in further work.
Despite the limitations, the current findings suggest that passive heat stress can impact the interhemispheric information interactions at resting state, and the VMHC deficits may play an important role in cognitive dysfunction. Our study may contribute to the understanding of neuromechanisms for psychosocial function impairments under heat stress.
Declaration of interest
The authors report no conflicts of interest. The authors thank all the participants for their time in completing the tasks. The authors alone are responsible for the content and writing of the paper.
This work was supported by the China Postdoctoral Science Foundation (no. 2014M552596).
References
- Jiang Q, Yang X, Liu K, Li B, Li L, Li M, et al. Hyperthermia impaired human visual short-term memory: An fMRI study. Int J Hyperthermia 2013;29:219–24
- Racinais S, Gaoua N, Grantham J. Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol 2008;586:4751–62
- Gaoua N, Racinais S, Grantham J, El Massioui F. Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia 2011;27:1–9
- Sun G, Yang X, Jiang Q, Liu K, Li B, Li L, et al. Hyperthermia impairs the executive function using the Attention Network Test. Int J Hyperthermia 2012;28:621–6
- Liu K, Sun G, Li B, Jiang Q, Yang X, Li M, et al. The impact of passive hyperthermia on human attention networks: An fMRI study. Behav Brain Res 2013;243C:220–30
- Sun G, Li L, Li M, Jiang Q. Hyperthermia impaired pre-attentive processing: An auditory MMN study. Neurosci Lett 2011;502:94–8
- Hancock PA, Vasmatzidis I. Effects of heat stress on cognitive performance: The current state of knowledge. Int J Hyperthermia 2003;19:355–72
- Sun G, Li M, Yang Z, Li L, Jiang Q, Zhao L. Hyperthermia exposure impaired the early stage of face recognition: An ERP study. Int J Hyperthermia 2012;28:605–20
- Banich MT, Belger A. Interhemispheric interaction: How do the hemispheres divide and conquer a task? Cortex 1990;26:77–94
- Davis SW, Kragel JE, Madden DJ, Cabeza R. The architecture of cross-hemispheric communication in the aging brain: Linking behavior to functional and structural connectivity. Cereb Cortex 2012;22:232–42
- Doron KW, Bassett DS, Gazzaniga MS. Dynamic network structure of interhemispheric coordination. Proc Natl Acad Sci USA 2012;109:18661–8
- Kreuter C, Kinsbourne M, Trevarthen C. Are deconnected cerebral hemispheres independent channels? A preliminary study of the effect of unilateral loading on bilateral finger tapping. Neuropsychologia 1972;10:453–61
- Hocking C, Silberstein RB, Lau WM, Stough C, Roberts W. Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A Mol Integr Physiol 2001;128:719–34
- Qian S, Sun G, Jiang Q, Liu K, Li B, Li M, et al. Altered topological patterns of large-scale brain functional networks during passive hyperthermia. Brain Cogn 2013;83:121–31
- Sun G, Qian S, Jiang Q, Liu K, Li B, Li M, et al. Hyperthermia-induced disruption of functional connectivity in the human brain network. PLoS One 2013;8:e61157
- Luo C, Li Q, Xia Y, Lei X, Xue K, Yao Z, et al. Resting state basal ganglia network in idiopathic generalized epilepsy. Hum Brain Mapp 2012;33:1279–94
- Ovadia-Caro S, Nir Y, Soddu A, Ramot M, Hesselmann G, Vanhaudenhuyse A, et al. Reduction in inter-hemispheric connectivity in disorders of consciousness. PLoS One 2012;7:e37238
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci 2008;28:13754–64
- Zuo X-N, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 2010;30:15034–43
- Hoptman MJ, Zuo XN, D’Angelo D, Mauro CJ, Butler PD, Milham MP, et al. Decreased interhemispheric coordination in schizophrenia: A resting state fMRI study. Schizophr Res 2012;141:1–7
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, et al. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry 2011;69:684–92
- Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, et al. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry 2013;41:24–9
- Guo W, Jiang J, Xiao C, Zhang Z, Zhang J, Yu L, et al. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr Res 2014;152:170–5
- Zhou G, Liu P, Zeng F, Yuan K, Yu D, von Deneen KM, et al. Increased interhemispheric resting-state functional connectivity in functional dyspepsia: A pilot study. NMR Biomed 2013;26:410–15
- Yuan K, Qin W, Liu P, Zhao L, Yu D, Dong M, et al. Reduced fractional anisotropy of corpus callosum modulates inter-hemispheric resting state functional connectivity in migraine patients without aura. PLoS One 2012;7:e45476
- Simons DJ, Rensink RA. Change blindness: Past, present, and future. Trends Cogn Sci 2005;9:16–20
- Lepsien J, Nobre AC. Attentional modulation of object representations in working memory. Cereb Cortex 2007;17:2072–83
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci 2001;5:119–26
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science 2001;291(5509):1803–6
- Emrich SM, Al-Aidroos N, Pratt J, Ferber S. Visual search elicits the electrophysiological marker of visual working memory. PLoS One 2009;4:e8042
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–8
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011;6:e25031
- Kitzbichler MG, Henson RN, Smith ML, Nathan PJ, Bullmore ET. Cognitive effort drives workspace configuration of human brain functional networks. J Neurosci 2011;31:8259–70
- Tao H, Guo S, Ge T, Kendrick KM, Xue Z, Liu Z, et al. Depression uncouples brain hate circuit. Mol Psychiatry 2013;18:101–11
- Calvert GA. Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb Cortex 2001;11:1110–23
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science 2000;288(5472):1769–72
- Henckens MJAG, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, et al. Stress-induced alterations in large-scale functional networks of the rodent brain. Neuroimage 2015;105:312–22
- Haag LM, Heba S, Lenz M, Glaubitz B, Hoffken O, Kalisch T, et al. Resting BOLD fluctuations in the primary somatosensory cortex correlate with tactile acuity. Cortex 2014;64C:20–8
- Powell TP, Mountcastle VB. Some aspects of the functional organization of the cortex of the postcentral gyrus of the monkey: A correlation of findings obtained in a single unit analysis with cytoarchitecture. Bull Johns Hopkins Hosp 1959;105:133–62
- Baddeley A. Working memory. Science 1992;255(5044):556–9
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature 1995;378(6554):279–81
- Funahashi S, Takeda K. Information processes in the primate prefrontal cortex in relation to working memory processes. Rev Neurosci 2002;13:313–45
- Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J Neurosci 2009;29:14980–6
- Yang ST, Shi Y, Wang Q, Peng JY, Li BM. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain 2014;7:1–13
- Qian S, Jiang Q, Liu K, Li B, Li M, Li L, et al. Effects of short-term environmental hyperthermia on patterns of cerebral blood flow. Physiol Behavior 2014;128:99–107
- Nunneley SA, Martin CC, Slauson JW, Hearon CM, Nickerson LD, Mason PA. Changes in regional cerebral metabolism during systemic hyperthermia in humans. J Appl Physiol (1985). 2002;92:846–51
- Fine EJ, Ionita CC, Lohr L. The history of the development of the cerebellar examination. Semin Neurol 2002;22:375–84
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science 1996;272(5261):545–7
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 2007;56:171–84
- Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL, Corbetta M. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci USA 2012;109:3516–21
- Guo W, Liu F, Liu Z, Gao K, Xiao C, Chen H, et al. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci Lett 2012;531:5–9
- Hu M, Li J, Eyler L, Guo X, Wei Q, Tang J, et al. Decreased left middle temporal gyrus volume in antipsychotic drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Schizophr Res 2013;144:37–42
- Schulte T, Muller-Oehring EM, Rohlfing T, Pfefferbaum A, Sullivan EV. White matter fiber degradation attenuates hemispheric asymmetry when integrating visuomotor information. J Neurosci 2010;30:12168–78
