Abstract
Purpose: The aim of this study was to assess the efficacy of definitive radiotherapy (RT) plus regional hyperthermia (HT) and investigate the potential contribution of HT to clinical outcomes in patients with prostate carcinoma. Materials and methods: Following our institution’s treatment protocol, HT was combined with RT to improve clinical outcomes in selected patients with high-risk or very high-risk prostate cancer. Data from 82 patients treated with RT plus HT and 64 patients treated with RT alone were retrospectively analysed. Results: Median follow-up duration was 61 months. The 5-year biochemical disease-free survival (bDFS) rate for the 82 patients treated with RT plus HT was 78%, whereas bDFS for the 64 patients treated with RT alone was 72%; this difference was not significant. Among the 75 patients treated with RT plus HT who underwent intra-rectal temperature measurements, higher thermal parameters were significant prognostic indicators of improved bDFS by univariate analysis. A higher CEM43 °CT90 thermal parameter and a T stage of T1–2 were significant prognostic factors based on multivariate analysis. The 5-year bDFS rates for the 40 patients with a higher CEM43 °CT90 and the 64 patients treated with RT alone were significantly different, whereas 5-year bDFS for the 35 patients with a lower CEM43 °CT90 and the 64 patients treated with RT alone were not. Conclusions: The addition of HT with higher thermal parameters to RT may improve bDFS for patients with high-risk or very high-risk prostate cancer. These findings also demonstrate the importance of careful selection of treatable patients with higher thermal parameters.
Introduction
High-risk prostate cancer is defined by the National Comprehensive Cancer Network (NCCN) as an elevated pretreatment serum prostate-specific antigen (PSA) value (>20 ng/mL), prostate biopsy with a high Gleason score (8–10), or either an advanced (T3a) or very high-risk (T3b or T4) clinical stage prostate cancer without lymph node metastasis. For patients with high-risk or very high-risk prostate cancer, external beam radiation therapy (RT) combined with androgen deprivation therapy (ADT) has traditionally been the primary treatment modality, producing a 5-year disease-free survival rate of 46–85%, in major previous clinical trials [Citation1,Citation2]. Thus, there is still room to further improve prostate cancer clinical outcomes.
Hyperthermia (HT) is known to be cytotoxic to cancer cells and acts as a radiation- and chemosensitiser [Citation3–7]. Radiation therapy-resistant tumour cells that are hypoxic, at a low pH, nutritionally deprived, and in S-phase are most sensitive to HT [Citation6]. Randomised, phase III clinical trials have shown the efficacy of RT plus HT in successfully treating patients with advanced head and neck cancer, locally recurrent breast cancer, malignant melanoma, bladder cancer, rectal cancer, cervical cancer, and glioblastoma [Citation6–9]. In experimental investigations the effects of radiosensitisation are substantial at temperatures of 42 °C or more [Citation10]. In addition, on the basis of the results of meta-analyses of breast tumour thermal data [Citation11], the local tumour control rates for patients with a superficial cancer were significantly better when tumours exhibited a higher intra-tumoural temperature and were treated with RT plus HT than when tumours had lower intra-tumoural temperatures [Citation10,Citation11]. Previous clinical phase I/II trials have confirmed that RT in combination with deep regional, interstitial, or transrectal ultrasound HT is promising and feasible without causing severe toxicity in patients with prostate cancer [Citation12–17]. In this context we added deep regional HT to RT to enhance the effects of RT in patients with high-risk or very high-risk prostate cancer, and hypothesised that there would be a positive relationship between clinical outcomes and tumour thermal parameters in patients. The purpose of this study was to assess the efficacy of RT combined with regional HT and the potential contribution of regional HT to clinical outcomes in patients with either high-risk or very high-risk prostate cancer.
Materials and methods
Patients
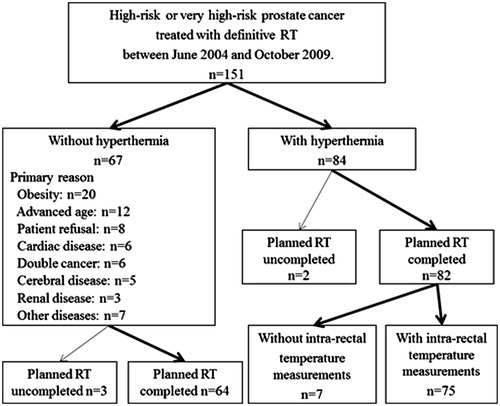
National Comprehensive Cancer Network (NCCN)-defined high-risk or very high-risk prostate cancer patients (n = 151) were treated with definitive RT between June 2004 and October 2009 at our institution. During the same period, according to our institution’s treatment protocol, regional HT was combined with definitive RT for a subset of patients (84/151; 56%) to potentially improve clinical outcomes (). The remaining 67 patients were treated with definitive RT alone. The primary indications against the use of regional HT were as follows: obesity (n = 20); advanced age (n = 12); patient refusal (n = 8); cardiac disease (n = 6); more than one concurrent cancer (n = 6); cerebral disease (n = 5); renal disease (n = 3); and the presence of other diseases (n = 7). Five of the 151 patients were not able to complete the planned RT dose and were excluded from the study. Therefore, 82 patients treated with definitive RT plus regional HT and 64 patients treated with definitive RT alone were included and retrospectively analysed (). The intra-rectal temperature was not measured for 7/82 patients treated with regional HT; therefore these subjects were excluded from the thermal analyses.
Patient baseline characteristics and treatments are listed in . All patients had pathologically confirmed prostate adenocarcinoma and initially underwent neoadjuvant ADT, median treatment time 9 months (2–39 months). Adjuvant ADT was continued for 20/146 (14%) patients after the completion of RT, median treatment time 5 months (1–40 months). Median total duration of neoadjuvant plus adjuvant ADT for all 146 patients was 10 months (2–70 months). Patients with post-operative prostate cancer were not included in this study. The study was approved by the Institutional Review Board of the University of Occupational Health and Environmental Health.
Table 1. The patient characteristics and treatment methods.
Radiotherapy
Radiation treatment was delivered to all patients with definitive intent. All patients were treated with a 10-MV linear accelerator using computed tomography (CT)-assisted three-dimensional (3D) conformal RT planning (FOCUS or Xio; CMS Japan, Tokyo, Japan) to determine the radiation fields for all patients, with treatment in the supine position. The total planned dose for all patients was 70 Gy, with a fractional dose of 2.0 Gy once a day (five times a week), except for the nine patients who were treated with RT alone (). The planned target volume (PTV) was delineated by contouring a clinical target volume (CTV) that included the entire prostate (plus seminal vesicles for cases with T3b or T4 grade tumours). Treatment included a PTV1 margin of 1.7 cm during the initial 46 Gy in 23 fractions and a PTV2 margin of 1.2 cm during the subsequent 24 Gy in 12 fractions, 20 Gy in 10 fractions, or 28 Gy in 14 fractions. The beams were shaped using a multileaf collimator. All patients were treated with 3D conformational or 7-field conformal radiation using an isocentric technique.
Hyperthermia
Heat was applied at 8 MHz radiofrequency-capacitive regional HT using a Thermotron RF-8 (Yamamoto Vinita, Osaka, Japan). The physical features of this instrument and the thermal distribution for a phantom model and the human body have been reported previously [Citation18–20]. Both the upper and lower electrodes measured 30 cm in diameter and were placed on opposite sides of the pelvis with the patient in the prone position. The treatment goal was at least 30 min of continuous heating after the radiofrequency output was increased to the patient’s tolerance threshold. Patients were carefully instructed to report any unpleasant sensations suggestive of a hot spot. The radiofrequency output was increased to the maximum level tolerated by the patient after appropriately adjusting the treatment setting. To reduce any preferential heating of subcutaneous fat tissue, overlay boluses were applied in addition to the regular boluses attached in front of the metal electrodes. The liquid (0.5% NaCl) inside the overlay boluses was cooled by the RF-8 circulatory system during heating.
HT was applied after RT once or twice a week. Median duration of heating was 50 min (30–50) in one heating session. The number of heating sessions ranged from two to six (median five) (). We directly measured the intra-rectal temperature in 75/82 patients using a four-point microthermocouple sensor. The sensor was inserted into the rectum at the level of the prostate and the intra-rectal temperature was measured during one (n = 56) or two (n = 19) HT treatments. The remaining seven patients did not have intra-rectal temperature assessed. The thermal parameters of the cumulative equivalent min at 43 °C for the T90 (CEM43 °CT90) and T50 were obtained on the basis of the intra-rectal temperatures during one session with temperature measurements obtained from 56 patients. Mean values were calculated separately for the 19 patients who underwent two measurements.
The T90 is an index temperature indicating when the target temperature was reached or surpassed by 90% of the intra-rectal measurement points, while the T50 indicates when the target temperature was reached or exceeded by 50% of the intra-rectal measurement points. The CEM43 °CT90 has been used extensively and successfully in clinical trials to assess the efficacy of heating [Citation21–23]. It represents the thermal isoeffect dose expressed in cumulative equivalent min at a reference temperature of 43 °C based on the low end of the temperature distribution (T90). The CEM43 °CT90 was calculated from the time-temperature data as follows:
When the temperature is higher than 43 °C, R = 0.5. When the temperature is lower than 43 °C, R = 0.25. In this protocol, ti is the time interval of the ith sample (ti = 1.0 min). The temperature exceeding the temperature at 90% of the intra-rectal measurement points during the ith minute was designated as T90i. We then used the CEM43 °CT90 to convert each T90i into an equivalent time at 43 °C. These equivalent times were then added together over the entire treatment duration of n min.
The isoeffect dose acquired over the whole treatment series (CEM43 °CT90tot) is the sum of the thermal dose per treatment with an intra-rectal temperature measurement (CEM43 °CT90), and was corrected for treatments for which no temperature measurement data were available as follows [Citation24]:
The ngiven is the number of treatments actually administered, and ndata is the number for which temperature data were available.
Follow-up
The length of follow-up was calculated from the RT start date. The patients were followed up at 1–3-month intervals during the first year and at 3–6-month intervals thereafter. At each follow-up visit, PSA was measured and assessments of potential gastrointestinal and genitourinary morbidity were performed. Biochemical failure was defined according to the Phoenix definition [Citation25]. The presence of bone metastasis was confirmed by bone scintigraphy, CT, or MRI. Soft tissue metastasis was confirmed on CT or MRI. The toxicity of therapy was evaluated according to the Common Terminology Criteria for Adverse Events version 3.0. The highest toxicity for each patient during and after RT with HT was entered into the toxicity analysis. Toxicity was defined as either acute (occurring during therapy or up to 3 months after therapy) or late (occurring more than 3 months after the completion of therapy).
Statistical analysis
Biochemical disease-free survival (bDFS), distant metastasis-free survival (DMFS), prostate cancer cause-specific survival, and overall survival (OS) rates were calculated starting from RT initiation using the Kaplan-Meier method. The statistical significance of the differences between the actuarial curves was assessed using the log-rank test. To identify prognostic factors for bDFS, univariate analyses were performed using the age, T stage, risk group, Gleason score, pretreatment PSA, length of ADT, and thermal parameters of the intra-rectal temperature. Multivariate analyses using a Cox proportional hazards model were performed to identify bDFS prognostic factors from among such factors as age, T stage, Gleason score, pretreatment PSA, length of ADT, and thermal parameters of the intra-rectal temperature. The chi-square test or Fisher's exact test was used to evaluate differences in clinical characteristics between patients with and without hyperthermia. The associations between certain factors, including the thermal parameters, performance status, patient age, thickness of the subcutaneous fat on CT images, and pelvic thickness on CT images were evaluated using linear regression analysis. The relationship between the CEM43 °CT90 of ≥1 min and the maximum anteroposterior diameter of the ventral subcutaneous fat was assessed using a Mann–Whitney U test. The Fisher's exact probability test was also used to compare Grade 2 or higher toxicity with the regional HT or CEM43 °CT90.
Results
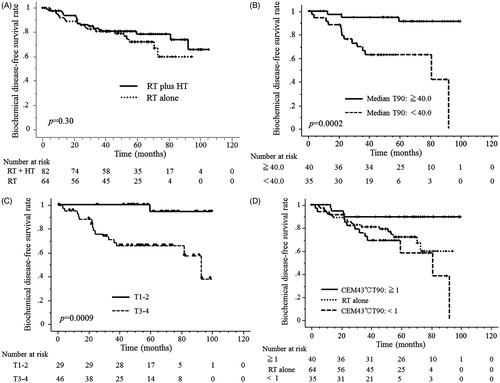
Median follow-up time was 61 months (1–105 months). The 5-year and 3-year bDFS rates were 81% and 75%, respectively, for all 146 patients. The 5-year and 3-year bDFS rates were 82% and 78% in 82 patients treated with RT plus HT and 81% and 72%, respectively, in 64 patients treated with RT alone; these differences were not significant (p = 0.30; ). The 5-year and 3-year bDFS rates in 75 patients treated with RT plus HT with the intra-rectal temperature measurements were 82% and 78%, respectively. The 5-year and 3-year DMFS rates were 96% and 91%, respectively, in all 146 patients. The 5-year and 3-year DMFS rates were 96% and 91% in 82 patients treated with RT plus HT and 95% and 91% in 64 patients treated with RT alone, respectively; these differences were not significant (p = 0.63). Finally, for all 146 patients, the 5-year and 3-year prostate cancer cause-specific survival rates were 99% and 98%, respectively, and the OS rates were 97% and 92%, respectively.
Figure 2. (A) There were no significant differences in bDFS between patient groups treated with RT plus HT or RT alone. (B, C) The thermal parameters of the higher median T90 (≥40.0 °C) and a T stage of T1–2 were found to be significant factors for predicting a better bDFS patient prognosis in the 75 patients who underwent intra-rectal temperature measurements. (D) The thermal parameters of the higher CEM43 °CT90 (≥1 min) were also significant for predicting a better bDFS patient prognosis in the 75 patients who underwent intra-rectal temperature measurements (p = 0.0021). The 5-year bDFS rate was 89.4% for the 40 patients with a higher CEM43 °CT90 (≥1 min) and 71.9% for the 64 patients treated with RT alone, which was significantly different (p = 0.0255). The 5-year bDFS rate was 57.9% in the 35 patients with a lower CEM43 °CT90 (<1 min) and 71.9% in the 64 patients treated with RT alone (p = 0.299).
The CEM43 °CT90 for the 75 patients who underwent intra-rectal temperature measurement ranged from 0.0 to 18.7 min, with a median of 1.2 min. There were 25 patients with a CEM43 °CT90 of < 0.5 min. The CEM43 °CT90tot ranged from 0.2 to 93.5 min, with a median time of 5.4 min. Median T90 values for each patient ranged from 36.9° to 44.0°C with a median of 40.0 °C among the 75 patients. In addition, the median T50 value for each patient ranged from 37.1 to 45.0 °C, with a median of 40.8 °C.
Among the same 75-patient cohort, univariate analyses showed that higher thermal parameters for the intra-rectal temperatures (CEM43 °CT90, T90, T50, and CEM43 °CT90tot), a T stage of T1–2, and NCCN high-risk prostate cancer were all significant predictors of a better prognosis for bDFS ( and ). In subsequent multivariate analyses for bDFS, a higher CEM43 °CT90 and a T stage of T1–2 were both significant factors predicting a better prognosis ().
Table 2. The results of the univariate analyses of factors predicting the biochemical disease-free survival in the 75 patients treated with definitive radiotherapy plus regional hyperthermia with intra-rectal temperature measurements.
Table 3. The results of the multivariate analyses of factors predicting the biochemical disease-free survival in the 75 patients who underwent intra-rectal temperature measurements.
Among the 56 patients treated with HT who underwent intra-rectal temperature measurements during only one heating session, univariate analyses showed that higher thermal parameters for the intra-rectal temperatures (CEM43 °CT90, T90, T50, and CEM43 °CT90tot) were also significant factors predicting a better prognosis for bDFS.
The 5-year bDFS rates for the 40 patients with a higher CEM43 °CT90 (≥1) and the 64 patients treated with RT alone were significantly different, whereas rates for the 35 patients with a lower CEM43 °CT90 (<1) and the 64 patients treated with RT alone were not different (). There was also no difference in bDFS between the 56 patients treated with RT alone that received totals of 70 Gy or 74 Gy and the eight patients treated with RT alone that received 66 Gy (p = 0.78).
Subcutaneous fat thicknesses at some levels were inversely correlated with the thermal parameters (). In particular, a significant negative correlation was observed between CEM43 °CT90 and the thickness of the maximum ventral subcutaneous fat in the pelvic region (). In addition, a CEM43 °CT90 of ≥1 min was significantly associated with a thinner maximum anteroposterior diameter of the ventral subcutaneous fat (p = 0.0036) (). Performance status and patients’ age were not correlated with the thermal parameters.
Figure 3. (A) The maximum anteroposterior diameters of the ventral subcutaneous fat were inversely correlated with CEM43 °CT90. (B) A significant relationship between a CEM43 °CT90 of ≥1 min and the maximum anteroposterior diameter of the ventral subcutaneous fat was also recognised (p = 0.0036).
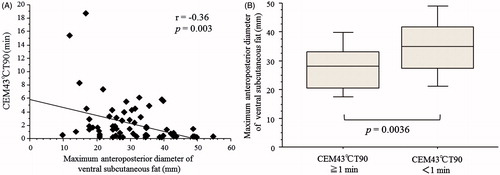
Table 4. The correlations between the intra-rectal thermal parameters and clinical characteristics.
The acute toxicities ≥Grade 2 recorded for patients treated with RT and regional HT were as follows: Grade 3 genitourinary (GU) toxicity in one patient (1%),and Grade 2 in 11 patients (13%), including Grade 2 gastrointestinal (GI) toxicity in two patients (2%). In patients treated with RT alone, the acute toxicities were as follows: Grade 3 GU toxicity in two patients (3%) and Grade 2 in six patients (10%), and Grade 3 GI toxicity in one patient (2%) and Grade 2 in one patient (2%). The occurrence of acute toxicities ≥Grade 2, including GU and GI, was not significantly different between patients with or without regional HT treatment. There were also no significant differences in the occurrence of GU or GI acute toxicities ≥Grade 2 between the 40 patients with a higher CEM43 °CT90 (≥1) and the 35 patients with a lower CEM43 °CT90 (<1). Skin burn presenting as a subcutaneous induration was observed in five patients, but spontaneously resolved after the completion of regional HT.
Late toxicities ≥Grade 2 in the patients treated with RT with regional HT were as follows: Grade 2 GI toxicity in 2 (2%) patients. The findings in the patients treated with RT alone were as follows: Grade 3 GI toxicity in one (2%) patient; and Grade 2 GU toxicity in 3 (5%) patients and Grade 2 in 2 (3%) patients. The occurrence of late toxicities ≥Grade 2 was not different between patients with or without regional HT treatment.
Discussion
The present study demonstrated that higher thermal parameters result in better bDFS for patients with high-risk or very high-risk prostate cancer treated with definitive RT plus regional HT. Many clinical phase III trials have demonstrated that adding HT to RT improves local control and complete response rates in patients with superficial tumours such as those involving head and neck cancer, recurrent breast cancer, and malignant melanoma [Citation11,Citation26,Citation27]. However, as previously mentioned, meta-analyses of the thermal data from four clinical phase III trials of recurrent breast cancer treated with RT, with or without HT, showed significant improvements in local control rates only in the patients who achieved higher intra-tumoural temperatures [Citation11]. Previous clinical studies of deep-seated tumours, including cervical cancer of the uterus, rectal cancer, and non-small cell lung cancer, that were treated with RT plus regional HT also demonstrated that thermal parameters correlate with clinical outcomes [Citation28–30]. In the current study, higher thermal parameters were found to be significant prognostic factors for better bDFS. This study also confirmed that regional HT with lower thermal parameters is insufficient to achieve the effects of radiosensitisation in patients with high-risk or very high-risk prostate cancer. These results may stimulate further technological development of deep regional HT.
The current study also demonstrates the importance of accurately selecting patients who are treatable with higher thermal parameters. One disadvantage of RF capacitive devices is the preferential heating of subcutaneous fat tissue. Asian patients are considered to be relatively suitable due to their slender constitution, whereas radiative phased array devices are more suitable for administering regional HT to Caucasian patients. Excessive power deposition in the fatty tissue limits the effectiveness of the capacitive technique and there is a depth limit to the skin-cooling ability of the overlay bolus in the 8-MHz RF capacitive heating device [Citation31,Citation32]. Thinner subcutaneous fat, a good performance status, and a younger age are significant predictive factors for successful deep regional heating using an RF capacitive device [Citation33]. Jones et al. conducted a randomised, phase III trial in patients with superficial cancer treated with RT, with or without HT [Citation22]. In their study, the patients received a test dose of HT and tumours deemed heatable (≥0.5 CEM43 °CT90) were randomly assigned to receive additional HT versus no additional HT [Citation22]. In the current study, higher thermal parameters, including the CEM43 °CT90, could also be achieved in patients with thinner subcutaneous fat. Future randomised trials that evaluate the addition of regional HT to RT in patients with high-risk or very high-risk prostate cancer should be designed to consider the selection of patients most likely to derive a benefit from HT.
Because obtaining direct intra-tumoural measurements of deep-seated pelvic tumours has the potential to cause severe complications (e.g. subcutaneous or deep infection, intolerable pain, and bleeding), intra-luminal thermometry (e.g. intravaginal, intra-rectal and intravesical thermometry) should be applied in cases of deep regional HT [Citation34]. Fatehi et al. reported that intra-luminal thermometry provides sufficient information for applying deep HT in individual patients with pelvic tumours, since the intra-tumoural and intra-luminal temperatures observed during individual treatment are highly correlated and the average intra-tumoural and intra-luminal temperatures do not differ [Citation35]. Therefore, in the current study, no intra-tumoural measurements were obtained, and the intra-rectal temperature at the level of the prostate was selected as representative for evaluation of the thermal parameters.
Obesity may promote the development of a more aggressive form of prostate cancer, thus resulting in higher recurrence rates after primary therapy [Citation36,Citation37]. Several potential biological mechanisms have been proposed to explain this link [Citation37]. However, the published literature does not uniformly support this unfavourable association, and the potential differences in biological properties thus remain controversial [Citation38–41]. In the current study, obesity-related factors such as the pelvic thickness and subcutaneous fat thickness were not significant predictors of bDFS in either the univariate or multivariate analyses, although significant negative correlations were observed between the thermal parameters and the thickness of the subcutaneous fat in the pelvic region. Therefore, we believe that the thermal parameters were independent predictive factors in patients treated with definitive RT plus regional HT for prostate cancer.
The present study was associated with several limitations. Intra-rectal temperatures were only measured during one (56 patients) or two (19 patients) treatments despite the administration of a median of five HT treatments, due to the extent of associated patient discomfort. Thus, it was not possible to provide a more complete thermal profile over the treatment course. De Bruijne et al. demonstrated that the methodology for extrapolating a thermal dose profile of over one or at most two treatments to an entire course must be viewed with extreme caution [Citation24]. After January 2012, intra-rectal temperature measurements were obtained for all of the hyperthermia treatments performed at our institution to more precisely evaluate the thermal dose. During these procedures, patient discomfort during the measurements was not found to be a significant issue for patient tolerance. Therefore, the current study indicated that a single (or at most double) treatment session with intra-rectal temperature measurements is sufficient for the evaluation of heatability, and that patients diagnosed with either high-risk or very high-risk prostate cancer who can be effectively heated will have better bDFS. Second, due to the fact that the study was retrospective, the possibility of selection bias with respect to the prognostic factors cannot be ruled out, although we performed both univariate and multivariate analyses for evaluating bDFS. A formal prospective trial is therefore needed to determine the efficacy and prognostic factors associated with this combined therapy in patients with high-risk or very high-risk prostate cancer.
In summary, this is the first report to assess the efficacy with respect to clinical outcomes of RT and regional HT combination therapy and the potential contribution of regional HT guided by intra-rectal temperature in patients with high-risk or very high-risk prostate cancer. Previous phase I/II clinical trials confirmed that RT with regional HT is promising and feasible without severe toxicity in patients with prostate cancer. In this study we demonstrated that the use of definitive RT combined with regional HT utilising higher thermal parameters is a promising treatment approach and that the application of regional HT with higher thermal parameters but not lower thermal parameters results in better bDFS. The importance of accurate selection of treatable patients with higher thermal parameters was also indicated. We found that the measurement of rectal temperatures for one (or at most two) treatment sessions was adequate for determining heatability. Higher thermal parameters may be achieved in patients with thinner subcutaneous fat in the pelvic region, and patients who could be effectively heated had a better 5-year bDFS. Our results provide justification for further evaluations in phase III, randomised clinical trials evaluating RT with or without regional HT in patients with higher thermal parameters and either high-risk or very high-risk prostate cancer.
Declaration of interest
The authors confirm that potential conflicts of interest do not exist in this study. The authors alone are responsible for the content and writing of the paper.
References
- Mohler JL, Armstrong AJ, Bahnson RR, Boston B, Busby JE, D’Amico AV, et al. Prostate cancer, Version 3.2012: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012;10:1081–7
- Payne H, Mason M. Androgen deprivation therapy as adjuvant/neoadjuvant to radiotherapy for high-risk localised and locally advanced prostate cancer: Recent developments. Br J Cancer 2011;105:1628–34
- Heijkoop ST, van Doorn HC, Stalpers LJ, Boere IA, van der Velden J, Franckena M, et al. Results of concurrent chemotherapy and hyperthermia in patients with recurrent cervical cancer after previous chemoradiation. Int J Hyperthermia 2014;30:6–10
- Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171–5
- Sousa A, Inman BA, Pineiro I, Monserrat V, Perez A, Aparici V, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia 2014;30:166–70
- Jones EL, Samulski TV, Leonard RP, Dewhirst MW: Hyperthermia, 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins, 2003
- van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002;13:1173–84
- Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin Oncol 2014;41:714–29
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Ben-Hur E, Elkind MM. Thermally enhanced radioresponse of cultured Chinese hamster cells: Damage and repair of single-stranded DNA and a DNA complex. Radiat Res 1974;59:484–95
- Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys 1997;39:371–80
- Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, et al. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: Long-term results from Dana-Farber Cancer Institute study 94–153. Cancer 2011;117:510–16
- Anscher MS, Samulski TV, Dodge R, Prosnitz LR, Dewhirst MW. Combined external beam irradiation and external regional hyperthermia for locally advanced adenocarcinoma of the prostate. Int J Radiat Oncol Biol Phys 1997;37:1059–65
- Van Vulpen M, De Leeuw AA, Raaymakers BW, Van Moorselaar RJ, Hofman P, Lagendijk JJ, et al. Radiotherapy and hyperthermia in the treatment of patients with locally advanced prostate cancer: Preliminary results. BJU Int 2004;93:36–41
- Tilly W, Gellermann J, Graf R, Hildebrandt B, Weissbach L, Budach V, et al. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol 2005;181:35–41
- Maluta S, Dall'Oglio S, Romano M, Marciai N, Pioli F, Giri MG, et al. Conformal radiotherapy plus local hyperthermia in patients affected by locally advanced high risk prostate cancer: Preliminary results of a prospective phase II study. Int J Hyperthermia 2007;23:451–6
- Deger S, Taymoorian K, Boehmer D, Schink T, Roigas J, Wille AH, et al. Thermoradiotherapy using interstitial self-regulating thermoseeds: An intermediate analysis of a phase II trial. Eur Urol 2004;45:574–9; discussion 580
- Song CW, Rhee JG, Lee CK, Levitt SH. Capacitive heating of phantom and human tumors with an 8 MHz radiofrequency applicator (Thermotron RF-8). Int J Radiat Oncol Biol Phys 1986;12:365–72
- Hiraoka M, Jo S, Akuta K, Nishimura Y, Takahashi M, Abe M. Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer 1987;60:121–7
- Abe M, Hiraoka M, Takahashi M, Egawa S, Matsuda C, Onoyama Y, et al. Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 1986;58:1589–95
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, et al. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993;25:289–97
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, et al. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005;23:3079–85
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94
- de Bruijne M, van der Holt B, van Rhoon GC, van der Zee J. Evaluation of CEM43 degrees CT90 thermal dose in superficial hyperthermia: A retrospective analysis. Strahlenther Onkol 2010;186:436–43
- Roach M III, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74
- Triantopoulou S, Efstathopoulos E, Platoni K, Uzunoglou N, Kelekis N, Kouloulias V. Radiotherapy in conjunction with superficial and intracavitary hyperthermia for the treatment of solid tumors: Survival and thermal parameters. Clin Transl Oncol 2013;15:95–105
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995;345(8949):540–3
- Franckena M, Fatehi D, de Bruijne M, Canters RA, van Norden Y, Mens JW, et al. Hyperthermia dose–effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer 2009;45:1969–78
- Ohguri T, Imada H, Yahara K, Morioka T, Nakano K, Terashima H, et al. Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer: The radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int J Radiat Oncol Biol Phys 2009;73:128–35
- Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, et al. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 2000;48:381–91
- Kroeze H, van de Kamer JB, de Leeuw AA, Kikuchi M, Lagendijk JJ. Treatment planning for capacitive regional hyperthermia. Int J Hyperthermia 2003;19:58–73
- Ohguri T, Imada H, Yahara K, Kakeda S, Tomimatsu A, Kato F, et al. Effect of 8-MHz radiofrequency-capacitive regional hyperthermia with strong superficial cooling for unresectable or recurrent colorectal cancer. Int J Hyperthermia 2004;20:465–75
- Ohguri T, Yahara K, Moon SD, Yamaguchi S, Imada H, Terashima H, et al. Deep regional hyperthermia for the whole thoracic region using 8 MHz radiofrequency-capacitive heating device: Relationship between the radiofrequency-output power and the intra-oesophageal temperature and predictive factors for a good heating in 59 patients. Int J Hyperthermia 2011;27:20–6
- van der Zee J, Peer-Valstar JN, Rietveld PJ, de Graaf-Strukowska L, van Rhoon GC. Practical limitations of interstitial thermometry during deep hyperthermia. Int J Radiat Oncol Biol Phys 1998;40:1205–12
- Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol 2007;183:479–86
- Stroup SP, Cullen J, Auge BK, L'Esperance JO, Kang SK. Effect of obesity on prostate-specific antigen recurrence after radiation therapy for localized prostate cancer as measured by the 2006 Radiation Therapy Oncology Group-American Society for Therapeutic Radiation and Oncology (RTOG-ASTRO) Phoenix consensus definition. Cancer 2007;110:1003–9
- Amling CL. Relationship between obesity and prostate cancer. Curr Opin Urol 2005;15:167–71
- Merrick GS, Galbreath RW, Butler WM, Wallner KE, Allen ZA, Adamovich E. Obesity is not predictive of overall survival following permanent prostate brachytherapy. Am J Clin Oncol 2007;30:588–96
- Davies BJ, Smaldone MC, Sadetsky N, Dall'era M, Carroll PR. The impact of obesity on overall and cancer specific survival in men with prostate cancer. J Urol 2009;182:112–17; discussion:117
- Geinitz H, Riegel MG, Thamm R, Astner ST, Lewerenz C, Zimmermann F, et al. Outcome after conformal salvage radiotherapy in patients with rising prostate-specific antigen levels after radical prostatectomy. Int J Radiat Oncol Biol Phys 2011;82:1930–7
- Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: Weighing the evidence. Eur Urol 2013;63:800–9