Abstract
Purpose: Glutathione constitutes the first line of the cellular defence mechanism against oxidative stress, and according to published data it is required by a number of factors that are involved in fever mechanism. The aim of the present study was to investigate whether or not glutathione deficiency can modulate a course of the fever induced by endotoxin (LPS). Material and methods: Intraperitoneal injection of LPS from Escherichia coli was used to provoke fever in Wistar rats. The level of liver glutathione was decreased by administration of phorone (Pho). Deep body temperature (Tb) in free running rats was recorded using a biotelemetry system. The concentration of TNF-α was estimated. Next, the supplementation of TNF-α was done using recombinant rat TNF-α. Results: Animals with decreased glutathione level responded with diminished fever after LPS injection (average Tb in Pho/LPS-treated and oil/LPS-treated animals were 36.90 ° ± 0.10 °C and 37.80 ° ± 0.15 °C, respectively). This response was accompanied by a significant attenuation of LPS-induced increase in TNF-α concentration (in the Pho/LPS-treated group it was 10.68 pg/mL ± 2.24, vs. 113.35 pg/mL ± 13.93 in oil/LPS-treated rats). Supplementation with TNF-α partially restored fever. Conclusion: Based on these data, we conclude that glutathione deficiency modifies the LPS-induced fever, in a TNF-α related manner.
Introduction
Fever is manifested by a rise in body temperature. It represents a physiological, highly regulated and adaptive response to pathological challenges such as infection, inflammation, injury and trauma [Citation1,Citation2,Citation3]. Infectious fever is triggered by a number of exogenous factors (i.e. exogenous pyrogens) of viral, bacterial, mycobacterial and parasitic origin [Citation4–10]. They include compounds such as polyinosinic polycytidylic acid (Poly-IC), lipopolysaccharides (LPS), peptidoglycans, porin complexes, lipoteichoic acid, lipoarabinomannans, bacterial and viral DNA and RNA, mycoplasma lipoproteins, staphylococcal and streptococcal proteinaceous superantigens. According to the current concept of fever physiology these exogenous factors do not act directly on the thermoregulatory centres to induce fever. They provoke synthesis of endogenous mediators of fever (i.e. endogenous pyrogens), which initiate a chain of the biochemical and physiological changes leading to the upward shifting of the thermoregulatory set-point. The most important mediators of fever are pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, interferons and tumour necrosis factor (TNF)-α [Citation11,Citation12].
Following a receptor-specific mechanism of the recognition of exogenous pyrogen structures, intracellular signalling pathways targeting on the activation of the transcriptional factor NF-kB are initiated [Citation13]. This factor is involved in regulation of the expression of a large number of mediators which participate in the acute inflammatory response, including pyrogenic cytokines, many of which are associated with increased generation of reactive oxygen species [Citation14]. We have documented [Citation9] that NF-kB plays a significant role in the generation of fever since mice lacking this factor (NF-kB gene knockout mice) appeared resistant to fever induced by the injection of LPS, a standard compound used to induce fever in the laboratory setting. There is also evidence indicating that modulation of NF-kB activation depends on the redox status of cells [Citation15,Citation16] and nuclear translocation of NF-kB can be triggered by exposure to H2O2 [Citation17]. Considering these data, one may suggest that redox status participates in modulation of fever response. In accordance, Riedel and co-workers [Citation18] have reported that inhibition of oxygen radical formation prevented LPS-induced fever in rabbits, and concluded that fever is a response to oxidative stress [Citation19].
The first line of the cellular defence mechanism against oxidative stress is glutathione-dependent. Glutathione is the major intracellular redox buffer in various cell types. It is essential for many responses of the immune system, including T lymphocyte proliferation [Citation20,Citation21], phagocytic activity of polymorphonuclear neutrophils [Citation22], dendritic cell functions [Citation23], and it is also important for the first step of the adaptive immunity, consisting of the antigen presentation by antigen presenting cells [Citation24]. It has been found that glutathione dynamically regulates functions of proteins, such as phosphatases, kinases and transcription factors [Citation25–27]. Accumulating evidence suggests that glutathione affects secretion of various cytokines, including those associated with fever and inflammation [Citation28]. For example, it has been shown that glutathione depletion in the murine antigen presenting cells decreased the expression of IL-12 and led to the polarisation from the typical pro-inflammatory and pro-febrile Th1 cytokine profile towards anti-inflammatory Th2 response patterns [Citation29]. Furthermore, Salzano and co-workers [Citation30] have postulated the involvement of glutathione in the secretion of TNF-α by macrophages treated with LPS. Since TNF-α is considered to be one of the earliest cytokines induced during endotoxic fever [Citation10], and because this cytokine plays a modulatory role at fever onset [Citation31], one may suggest that glutathione takes part in the mechanism of fever.
The main aim of this article was to examine the hypothesis that glutathione modulates fever following LPS administration, and that this kind of feverish response is reversed by TNF-α.
Materials and methods
A total of 60 specific, pathogen-free male Wistar rats weighing 200–250 g were used throughout the experimentation. The exact number of animals in each experiment is provided in figures. Rats were obtained from Experimental and Clinical Medical Institute Warsaw (Poland) and were housed in individual plastic cages and placed in a temperature/humidity/light-controlled chamber set at 22 ° ± 1 °C, 12:12 h light:dark cycle, with light on at 07:00 h. Rodent laboratory food and drinking water were provided ad libitum. One week after shipping, the rats were implanted with biotelemetry devices to monitor deep body temperature. Rats showing a regular and stable 24-h body mass gain were taken to the experiments. All experimental procedures were approved by the Local Bioethical Committee for Animal Care (permission no. 8/2011).
Body temperature measurement
Deep body temperature (Tb) of the rats was measured using battery-operated telemetry transmitters (model TA-F40, Data Sciences International, St Paul, MN) implanted intra-abdominally under sterile conditions. Before the implantation the rats were anaesthetised with a mixture of ketamine (87 mg/kg) (Biowet, Drwalew, Poland) and xylazine (13 mg/kg) (ScanVet, Warsaw, Poland) injected intramuscularly. Then, following shaving and sterilisation of a small abdominal surgical area, an incision was made in the skin and muscles of the abdomen, and a miniature temperature-sensitive telemetry device was introduced into the peritoneal cavity. The muscle level of the abdomen and the skin were separately sutured closed. All experiments started 10 days after recovery from the surgery.
Induction of LPS fever in the telemetry-implanted rats
Lipopolysaccharide (LPS) extracted from Escherichia coli 0111:B4 (Sigma-Aldrich, St Louis MO) was dissolved in sterile 0.9% sodium chloride (saline). Before injection, the stock solution of LPS (2.5 mg/mL) was warmed to 37 °C, diluted in warm sterile saline to the desired concentration, and injected intraperitoneally (i.p.) at a dose of 50 μg/kg, as described previously [Citation9,Citation24]. Control rats were injected i.p. with an equivalent volume of pyrogen-free saline.
Phorone and TNF-α preparation and administration
Phorone (Pho), a well-known glutathione depleting agent [Citation32–34] was purchased from Sigma-Aldrich. It was dissolved in olive oil and injected i.p. at a dose of 80 mg/kg 2 h prior to LPS challenge. Olive oil without Pho at an equivalent volume was used as the control injection. Before injection, both Pho solution and oil were warmed to 37 °C. The effect of Pho on the normal circadian rhythm in Tb of the rats was also evaluated and compared to the non-treated free-running rats.
Recombinant rat TNF-α (PeproTech, Rocky Hill, NJUSA) in a phosphate buffered saline (PBS, pH 7.4) was administered i.p. at a dose of 5 µg/kg 2 h prior to the injection of LPS. PBS in an equivalent volume was used as a control injection.
To perform all injections described in this section, as well as in the previous one, the rats were briefly restrained and then were placed in their home cages immediately afterwards.
Liver glutathione assay
Liver samples (100 mg) were dissected out from anaesthetised Wistar rats 3 h after LPS injection. Next, they were rinsed twice with PBS and homogenised on ice in 1 mL of 5% trichloroacetic acid (PoCh, Gliwice, Poland). Total glutathione contents were determined using a glutathione assay kit (Sigma-Aldrich) according to the manufacturer’s protocol. Samples were assayed in triplicate. Colorimetric changes in the assays were detected using a plate reader (BioTek Instruments, Winooski, VT).
Plasma TNF-α assay
Blood samples for cytokine analysis were collected from rats by cardiac puncture at 3 h after LPS injection as described in the previous section. Blood was drawn into EDTA tubes and plasma was separated by centrifugation (20 min at 1000 × g). Plasma was stored at−20 °C until assay.
TNF-α levels were determined in a plate reader (Bio-Tek Instruments) using a highly sensitive colorimetric assay kit from R&D Systems (Minneapolis, MN) according to the manufacturer’s instruction. Samples were assayed in triplicate.
Data analysis
Temperature data are expressed as means ± SE. Injections were performed at time points indicated in figures. Data were recorded and computed at 5-min intervals using Dataquest ART System (Data Sciences International). For data analysis, excel plotting and presentation, the temperature recordings were pooled into 30-min averages.
One-way ANOVA was used to analyse significance of changes in the body temperature of the rats, concentration of glutathione in the liver and TNF-α in plasma. As a post hoc test the Tukey-Kramer (HSD) test was used. The threshold of statistical significance was p < 0.05 for all tests.
Results
Changes of the rat liver glutathione content following treatment with phorone and LPS
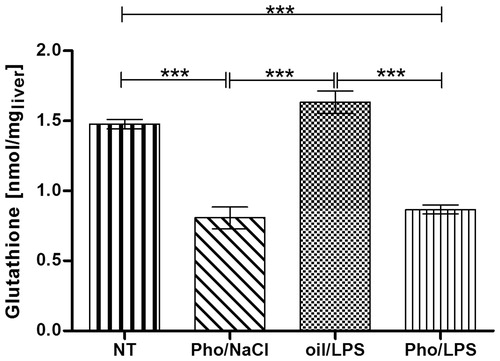
The concentration of glutathione was assayed to check whether Pho in a dose of 80 mg/kg reduced its level in rat’s liver. shows that glutathione concentration was affected by Pho administration (F3.12 = 48.44, p < 0.001) (one-way ANOVA). In detail, Pho administration resulted in a significant (p < 0.001) decrease of the liver glutathione concentration in both Pho/LPS and Pho/NaCl groups in comparison with that recorded in non-treated (NT) rats (Pho/LPS 0.87 ± 0.03 nmol/mg and Pho/NaCl 0.80 ± 0.08 nmol/mg vs. 1.48 ± 0.03 nmol/mg in the NT group). The concentration in the group of rats treated with oil/LPS was 1.63 ± 0.08 nmol/mg, and was significantly higher than those of both phorone-treated groups (p < 0.001). A slight increase in the liver glutathione concentration in the oil/LPS group (1.63 ± 0.08 nmol/mg vs. 1.48 ± 0.03 nmol/mg in NT group) was insignificant (p < 0.313).
Figure 1. Alterations of glutathione concentrations in the liver of rats injected at 07:00 h with phorone (Pho) or olive oil and at 09:00 h with saline (NaCl) or endotoxin (LPS). The livers were collected at 12:00 h. NT indicates the non-treated group. Each group of animals consisted of four rats. Asterisks indicate significant differences (***p < 0.001).
Effect of phorone on LPS-induced fever
The role of glutathione in fever was checked by measurement of body temperature (Tb) in Pho-treated rats.
A standard LPS-induced fever has been described previously [Citation35]. Briefly, intraperitoneal injection of the saline suspension of LPS at a dose of 50 µg/kg induced biphasic fever (). Fever started within 2 h after LPS injection, and the first peak of body temperature was reached and maintained during the next 1.5 h (the average Tb was 37.80 ° ± 0.17 °C in oil/LPS-treated rats vs. 37.40 ° ± 0.07 °C in NT animals) (p < 0.01). The second peak of fever was reached within 4.5 h post-injection and maintained for the following 2.0 h (average Tb for the oil/LPS-treated animals was 38.20 ° ± 0.07 °C and for NT animals was 37.60 ° ± 0.01 °C during this time) (p < 0.001). Then there followed a 4-hgradual decrease of Tb towards its normal value.
Figure 2. Changes of body temperature of rats treated at 07:00 h (arrowed) with Pho at a dose of 80 mg/kg or with equivalent volume of oil and then at 09:00 h injected with LPS at a dose of 50 μg/kg (arrowed) vs. non-treated rats (NT). Values are means ± SE of 30-min averages. Black horizontal line shows lights-off period in a 12:12-h light dark cycle.
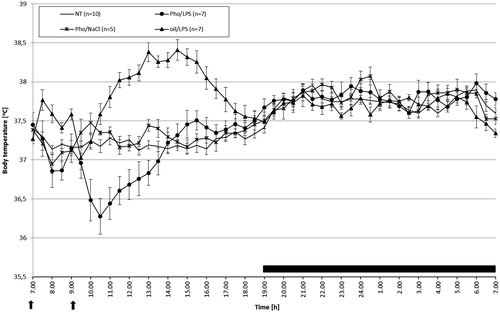
Injection of Pho resulted in a drop of Tb that was enhanced after LPS administration (average Tb over a 7-h period from 07:00 to 14:00 h for the Pho/LPS-treated, oil/LPS-treated, Pho/NaCl-treated and NT animals was 36.90 ° ± 0.11 °C, 37.80 ° ± 0.16 °C, 37.30 ° ± 0.07 °C and 37.30 ° ± 0.06 °C, respectively). Analysis of variance (experimental group as factor: F3.56 = 25.01, p < 0.001) showed that body temperature of rats treated with Pho/LPS was significantly lower than that recorded in oil/LPS (p < 0.001), Pho/NaCl (p < 0.001) and NT-treated (p < 0.01) rats.
Next, the body temperature of rats treated with Pho/LPS was still significantly different (p < 0.01) than that recorded in the NT group (within the 5-h period from 14:00 to 19:00 h Tb of Pho/LPS-treated, Pho/NaCl-treated, and NT animals was 37.40 ° ± 0.05 °C, 37.30 ° ± 0.05 °C, and 37.30 ° ± 0.03 °C, respectively).
Night-time body temperature of rats treated with Pho and LPS did not differ significantly from that in NT rats (p < 0.32) and Pho/NaCl rats (p < 0.62), but was significantly higher than that recorded in oil/LPS group (p < 0.001) (12-h average Tb for Pho/LPS-treated, oil/LPS-treated, and NT animals was 37.80 ° ± 0.03 °C; 37.70 ° ± 0.05 °C, and 37.80 ° ± 0.05 °C, respectively).
Effect of phorone and LPS on plasma TNF-α
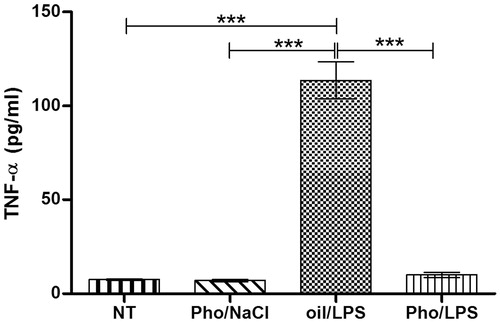
TNF-α is considered the first proinflammatory cytokine induced after LPS injection. We investigated whether fever inhibition in glutathione-depleted rats is associated with this cytokine. Indeed, one-way ANOVA showed that the concentration of TNF-α () was strongly affected in the experimental group (F 3.12 = 111.97, p < 0.001). The highest concentration of TNF-α in plasma (113.58 ± 9.84 pg/mL) was observed in oil/LPS-treated rats and was significantly different (p < 0.001) than those recorded in Pho/LPS-treated, Pho/NaCl-treated, and NT rats (10.06 ± 1.31 pg/mL, 7.03 ± 0.62 pg/mL, and 7.51 ± 0.42 pg/mL, respectively).
Figure 3. The effect of Pho on plasma TNF-α level in animals treated with LPS vs. Pho/NaCl, oil/LPS and non-treated rats (NT). Pho and LPS were injected at 07:00 h and at 09:00 h, respectively. Plasma was collected at 12:00 noon. Each group of animals consisted of four rats. Asterisks indicate significant difference (***p < 0.001) in the legend of .
Effect of TNF-α injection on body temperature of rats treated with phorone and LPS
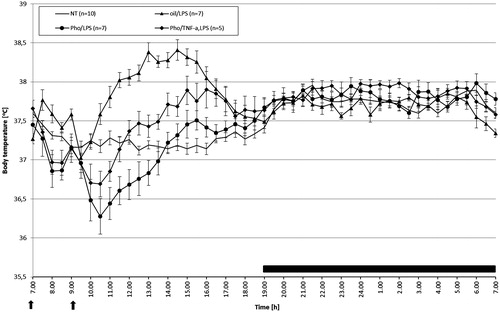
To check whether antipyretic properties of Pho are associated with decreased level of TNF-α we treated rats with 5 µg/kg of this cytokine (). One-way ANOVA (F 2.42 = 7.83, p < 0.001) showed that at the beginning of the experiment (from 07:00 to 14:00 h) body temperature of Pho/TNF-α-treated and LPS-treated rats differed significantly from those recorded in Pho/LPS-treated and oil/LPS-treated rats, but did not differ from body temperature recorded in NT rats (p < 0.99) (average Tb for Pho/TNF-α-treated, LPS-treated, Pho/LPS-treated, oil/LPS-treated, and NT rats was 37.20 ° ± 0.15 °C, 36.90 ° ± 0.11 °C, 37.80 ° ± 0.16 °C, and 37.30 ° ± 0.06 °C, respectively). In the following hours (from 14:00 to 19:00 h) body temperature of Pho/TNF-α, LPS-treated rats was still higher than that in Pho/LPS-treated rats (p < 0.001) and higher compared with that recorded in their NT counterparts (p < 0.001), but did not differ significantly from that recorded in the oil/LPS-treated group (p < 0.99) (average Tb for Pho/TNF-α-treated, LPS-treated, Pho/LPS-treated, oil/LPS-treated, and NT rats was 37.70 ° ± 0.05 °C, 37.40 ° ± 0.05 °C, 37.90 ° ± 0.13 °C and 37.30 ° ± 0.03 °C, respectively). Night-time body temperatures did not differ significantly.
Figure 4. Changes of body temperature of rats treated i.p. with Pho (80 mg/kg) at 07:00 h and then at 09:00 h injected with LPS at a dose of 50 μg/kg or LPS and TNF-α at a dose of 5 μg /kg (arrowed). Values are means ± SE of 30-min averages. Black horizontal line shows lights-off period in a 12:12-h light-dark cycle.
Discussion
In the present study, we demonstrated that low availability of glutathione contributes to fever reduction in rats. Furthermore, we showed that the inhibition of fever was accompanied by a decrease in TNF-α level, and was partially restored by an injection of TNF-α.
There is a growing body of evidence that reactive oxygen species are involved in fever mechanism [Citation18,Citation19]. One of the earliest events after LPS entry into the circulatory system seems to be the production of oxygen radicals by various cells including Kupffer cells and macrophages [Citation36–38]. Pretreatment with free radical scavengers significantly attenuates both fever and increase in hypothalamic level of hydroxyl radicals following systemic administration of LPS [Citation39]. These results are fully comparable with ours [Citation35] in which we observed antipyretic properties of a commonly used antioxidant such as N-acetylcysteine.
Glutathione is a molecule that is produced in all organs, especially in the liver, and its depletion occurs under several pathological conditions, including long-term ethanol intake [Citation40], cerebral ischaemia [Citation41], exposure to environmental toxins [Citation42], and cancer [Citation43]. Decreased glutathione level results in increased accumulation of reactive oxygen species, impaired immune responses, inflammation, and increased susceptibility to infection [Citation22]. Reactive oxygen species are continuously generated in various cellular processes, and the imbalance between their production and the available anti-oxidative defence against them leads to oxidative stress [Citation44]. Because glutathione has many important physiological and metabolic functions, we tried to answer a question: are organisms with reduced levels of glutathione able to develop fever after endotoxin administration?
In the current experiments, deprivation of glutathione was achieved by administration of phorone. Phorone is an α,β-unsaturated carbonyl compound, that preferentially depletes hepatic glutathione via enzymatic conjugation by glutathione-transferases, followed by biliary excretion of these conjugates [Citation45,Citation46]. Although phorone is widely used as a glutathione depleter [Citation32–34], we cannot exclude that it may affect fever via glutathione independent mechanisms. Therefore, in a future study we are going to investigate fever in phorone-treated animals having liver glutathione restored by administration of glutathione precursors.
According to published data, one may assume that oxidative stress, which appears as a consequence of glutathione depletion, is intensified after LPS administration [Citation36–38]. As free radical scavengers such as methylene blue and N-acetylcysteine inhibit fever [Citation18,Citation24], glutathione depleters should enhance it. However, contrary to these expectations, in the present experiments, we were able to show a significant inhibition of fever in rats with decreased glutathione level. This finding might explain the reason why many cancer patients lack infectious fever [Citation47–49].
The mechanisms by which deficiency of glutathione affects fever are still unknown. In our previous paper [Citation9] we have shown that NF-kB is crucial for fever induction because NF-kB-deficient mice did not respond with fever after endotoxin injection. There are some experimental data that activation of NF-kB is glutathione-regulated [Citation50,Citation51]. It is also well documented that glutathione depletion induces haem oxygenase-1 (HO-1) [Citation52–56]. Induction of HO-1 is considered an adaptive and protective response to oxidative stress [Citation57]. Moreover, reduced levels of the proinflammatory cytokines such as TNF-α and IL-6 were detected after HO-1 induction [Citation58].
Glutathione may also modulate fever by affecting the synthesis of prostaglandin E2 (PGE2), which is the final mediator of the febrile response. PGE2 production requires an increase in activity of membrane-associated PGE synthase-1 (mPGES-1) [Citation59]. No activity of synthase mPGES-1 was detected in the absence of glutathione [Citation60].
As we mentioned above, induction of fever is mediated by the release of cytokines [Citation11,Citation12]. TNF-α is considered the first proinflammatory cytokine induced after LPS injection [Citation10,Citation31,Citation61,Citation62]. Intracerebral administration of neutralising antibodies to TNF-α causes attenuation to the febrile response to systemic injection of LPS [Citation63]. In the present study, we examined whether glutathione deficiency affects LPS-induced TNF-α level in rats. Indeed, these animals released less TNF-α than those maintaining physiological level of glutathione. It has been shown that regulation of LPS-induced TNF-α biosynthesis is redox-sensitive and requires the involvement of glutathione-mediated signalling pathways [Citation28].
The results of the current investigation confirm our assumption that glutathione deficiency affects body temperature following the LPS administration. Interestingly, rats depleted of glutathione did not show fever that was accompanied by a plasma TNF-α reduction. Because supplementation with TNF-α only partially restored feverish response in glutathione depleted animals, we suppose that other factors are also likely to be involved in the observed fever inhibition.
It is known that fever following infection and inflammation is beneficial for healing processes [Citation64]. On the other hand, fever is associated with oxidative stress [Citation19]. Therefore each type of cells has precisely regulated antioxidative protection, in which glutathione plays a crucial role. We speculate that depriving organisms of this natural protection would lead to domination of adverse effects of fever over its benefits. Under such conditions, organisms do not develop fever. However, this issue needs further research.
Declaration of interest
This study was supported by grant no. 2012/07/B/NZ4/00197/ from the Polish National Science Centre. The authors alone are responsible for the content and writing of the paper.
References
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszyński D. The adaptive value of fever. In: Mackowiak PA, editor Fever: Basic Mechanisms and Management, 2nd ed. Philadelphia: Lippincott-Raven, 1997. pp 255–66
- Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis 2002;15:241–5
- Singh IS, Hasday JD. Fever, hyperthermia and the heat shock response. Int J Hyperthermia 2013;29:423–35
- Kozak W, Conn CA, Kluger MJ. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol 1994;266:R125–35
- DalNogare AR, Sharma S. Exogenous pyrogens. In: Mackowiak PA, editor Fever: Basic Mechanisms and Management, 2nd ed. Philadelphia: Lippincott-Raven, 1997. pp 79–86
- Galdiero F, Sommese L, Scarfogliero P, Galdiero M. Biological activities lethality, Shwartzman reaction and pyrogenicity of Salmonella typhimurium porins. Microb Pathog 1994;16:111–19
- Moesby L, Hansen EW, Christensen JD, Tommerup L, Nielsen C. Endospores of B. subtilis are pyrogenic and activate Mono Mac 6 cells: importance of the CD14 receptor. Eur J Pharm Sci 2003;19:245–51
- Proft T, Fraser JD. Bacterial superantigens. Clin Exp Immunol 2003;133:299–306
- Kozak W, Wrotek S, Kozak A. Pyrogenicity of CpG-DNA in mice: Role of interleukin-6, cyclooxygenases, and nuclear-factor-kappa-B. Am J Physiol 2006;290(4):R871–80
- Roth J, Blatteis CM. Mechanisms of fever production and lysis: lessons from experimental LPS fever. Comp Physiol 2014;4:1563–604
- Blatteis CM, Li S, Li Z, Feledr C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostagl Lipid Mediat 2005;76:1–18
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci 2004;1:1433–49
- Lawrence T. The nuclear factor NF-kappa B pathway in inflammation. Cold Spring Harb Perspect Biol 2009;1:a001651
- Abraham E. Nuclear factor kB and its role in sepsis-associated organ failure. J Infect Dis 2003;187:S364–9
- Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kB activation. Free Radical Biol Med 1997;22: 1115–26
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kB. J Immunol 2004;172:2522–9
- Janssen-Heiniger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kB. Free Radical Biol Med 2000;28:1317–27
- Riedel W, Lang U, Oetjen U, Shlapp U, Shibata M. Inhibition of oxygen radical formation by methylene blue, aspirin or a-lipoic acid, prevents bacterial-lipopolysaccharide-induced fever. Mol Cell Biochem 2003;247:83–94
- Riedel W, Maulik G. Fever: an integrative response of the central nervous system to oxidative stress. Mol Cell Biochem 1999;196:125–32
- Sido B, Braunstein J, Breitkreutz R, Herfarth C, Meuer SC. Thiol-mediated redox regulation of intestinal lamina propria T lymphocytes. J Exp Med 2000;192:907–12
- Hadzic T, Li L, Cheng N, Walsh SA, Spitz DR, Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol 2005;175:7965–72
- Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J Gen Med 2011;4:105–13
- Kuppner MC, Scharner A, Milani V, Von Hesler C, Tschop KE, Heinz O, et al. Ifosfamide impairs the allostimulatory capacity of human dendritic cells by intracellular glutathione depletion. Blood 2003;102:3668–74
- Wrotek S, Jedrzejewski T, Potera-Kram E, Kozak W. Antipyretic activity of N-acetylcysteine. J Physiol Pharmacol 2011;62:669–75
- Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, et al. Redox modulation of cell surface protein thiols in U937 lymphoma cells: the role of g-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med 1999;27:623–35
- Biswas S, Chida AS, Rahman I. Redox modifications of proteinthiols: emerging roles in cell signaling. Biochem Pharmacol 2006;71:551–64
- Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 2005;7:42–59
- Haddad JJ, Land SC. Redox/ROS regulation of lipopolysaccharide-induced mitogen-activated protein kinase (MAPK) activation and MAPK-mediated TNF-alpha biosynthesis. Br J Pharmacol 2002;135:520–36
- Dobashi K, Aihara M, Araki T, Shimizu Y, Utsugi M, Iizuka K, et al. Regulation of LPS induced IL-12 production by IFN-gamma and IL-4 through intracellular glutathione status in human alveolar macrophages. Clin Exp Immunol 2001;124:290–6
- Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci USA 2014;111:12157–62
- Kozak W, Conn CA, Klir JJ, Wong GH, Kluger MJ. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol 1995;269:R23–9
- Negi B, Kaur R, Dey G. Protective effects of a novel sea buckthorn wine on oxidative stress and hypercholesterolemia. Food Funct 2013;4:240–8
- Gao W, Mizukawa Y, Nakatsu N, Minowa Y, Yamada H, Ohno Y, et al. Mechanism-based biomarker gene sets for glutathione depletion-related hepatotoxicity in rats. Toxicol Appl Pharmacol 2010;247:211–21
- Jung YS, Kim SJ, Kwon do Y, Kim YC. Comparison of the effects of buthioninesulfoximine and phorone on the metabolism of sulfur-containing amino acids in rat liver. Biochem Biophys Res Commun 2008;368:913–18
- Wrotek S, Jedrzejewski T, Potera-Kram E, Kozak W. Antipyretic activity of N-acetylcysteine. J Physiol Pharmacol 2011;62:669–75
- Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-α production. J Leukoc Biol 2006;79:1348–56
- Rossi F. The 02-forming NADPH oxidase of the phagocytes: Nature, mechanisms of activation and function. Biochim Biophys Acta 1986;853:65–89
- Landmann R, Scherer F, Schumann R, Link S, Sansano S, Zimmerli W. LPS directly induces oxygen radical production in human monocytes via LPS binding protein and CD14. J Leukoc Biol 1995;57:440–9
- Weihrauch D, Riedel W. Nitric oxide (NO) and oxygen radicals, but not prostaglandins, modulate fever. Ann N Y Acad Sci 1997;813:373–82
- Hirano T, Kaplowitz N, Tsukamoto H, Kamimura S, Fernandez-Checa JC. Hepatic mitochondrial glutathione depletion and progression of experimental alcoholic liver disease in rats. Hepatology 1992;16:1423–7
- Anderson MF, Sims NR. The effects of focal ischemia and reperfusion on the glutathione content of mitochondria from rat brain subregions. J Neurochem 2002;81:541–9
- Nigam D, Shukla GS, Agarwal AK. Glutathione depletion and oxidative damage in mitochondria following exposure to cadmium in rat liver and kidney. Toxicol Lett 1999;106:151–7
- Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother 2003;57:145–55
- Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia 2014;30:513–23
- Aquilano K, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signalling for an old antioxidant. Front Pharmacol 2014;5:196
- Plummer JL, Smith BR, Sies H, Bend JR. Chemical depletion of glutathione in vivo. Methods Enzymol 1981;77:50–9
- Wrotek S, Kamecki K, Kwiatkowski S, Kozak W. Cancer patients report a history of fewer fevers during infections than healthy controls. J Pre-Clin Clin Res 2009;3:31–5
- Grossarth-Maticek R, Frentzel-Beyme R, Kanazir D, Jankovic M, Vetter H. Reported herpes-virus infection, fever and cancer incidence in a prospective study. J Chronic Dis 1987;40:967–76
- Kolmel K, Gefeller O, Haverkamp B. Febrile infections and malignant melanoma: results of a case-control study. Melanoma Res 1992;2:207–11
- Kil IS, Kim SY, Park JW. Glutathionylation regulates IkB. Biochem Biophys Res Commun 2008;373:169–73
- Seidel P, Merfort I, Hughes JM, Oliver BG, Tamm M, Roth M. Dimethylfumarate inhibits NF-kB function at multiple levels to limit airway smooth muscle cell cytokine secretion. Am J Physiol 2009;297:L326–39
- Thiessen A, Schmidt MM, Dringen R. Fumaric acid dialkyl esters deprive cultured rat oligodendroglial cells of glutathione and upregulate the expression of heme oxygenase 1. Neurosci Lett 2010;475:56–60
- Lehmann JC, Listopad JJ, Rentzsch CU, Igney FH, von Bonin A, Hennekes HH, et al. Dimethylfumarate induces immunosuppression via glutathione depletion and subsequent induction of heme oxygenase 1. J Invest Dermatol 2007;127:835–45
- Saunders EL, Maines MD, Meredith MJ, Freeman ML. Enhancement of heme oxygenase-1 synthesis by glutathione depletion in Chinese hamster ovary cells. Arch Biochem Biophys 1991;288:368–73
- Ewing JF, Maines MD. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem 1993;60:1512–19
- Horikawa S, Yoneya R, Nagashima Y, Hagiwara K, Ozasa H. Prior induction of heme oxygenase-1 with glutathione depletor ameliorates the renal ischemia and reperfusion injury in the rat. FEBS Lett 2002;510:221–4
- Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol 2010;80:1895–903
- Tu TH, Joe Y, Choi HS, Chung HT, Yu R. Induction of heme oxygenase-1 with hemin reduces obesity-induced adipose tissue inflammation via adipose macrophage phenotype switching. Mediators Inflamm 2014;2014:290708
- Saha S, Engström L, Mackerlova L, Jakobsson PJ, and Blomqvist A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol 2005;288:R1100–7
- Thorén S, Jakobsson PJ. Coordinate up- and down-regulation of glutathione-dependent prostaglandin E synthase and cyclooxygenase-2 in A549 cells. Inhibition by NS-398 and leukotriene C4. Eur J Biochem 2000;267:6428–34
- Roth J, Conn CA, Kluger MJ, Zeisberger E. Kinetics of systemic and intrahypothalamic IL-6 and tumour necrosis factor during endotoxin fever in guinea pigs. Am J Physiol 1993;265:R653–8
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev 1991;71:93–127
- Roth J, Martin D, Störr B, Zeisberger E. Neutralization of pyrogen-induced tumour necrosis factor by its type 1 soluble receptor in guinea pigs: effects on fever and interleukin-6 release. J Physiol 1998;509:267–75
- El-Radhi AS. Fever management: Evidence vs current practice. World J Clin Pediatr 2012;1:29–33
