Abstract
Purpose: The aim of this study was to elucidate the clinical significance of preoperative Platelet-to-lymphocyte ratio (PLR) in recurrent hepatocellular carcinoma (RHCC) patients after thermal ablation.
Materials and methods: We retrospectively reviewed 414 patients with RHCC treated with ultrasound-guided thermal ablation percutaneously between January 2010 and March 2014. The correlation of recurrence-free survival (RFS) with 15 clinical parameters was analysed by Cox multivariate proportional hazard model analysis. The best cut-off value of preoperative PLR was determined with time-dependent receiver operating characteristic (ROC) curve analysis. The value of PLR in predicting recurrence was analysed by Kaplan-Meier.
Results: Multivariate Cox proportional hazard model analysis showed that tumour differentiation, prothrombin time (PT), absolute lymphocyte count (ALC) and PLR were risk factors for recurrence in RHCC patients. PLR ≥ 87.87 was considered for evaluation (AUROC = 0.667; P < 0.05), and 166 of 414 patients (40.1%) had PLR of more than 87.87. During the follow-up period (12–52 months), the 1- and 3-year recurrence rates were 39.9% and 54.8% in the low PLR group, which were significantly better than those in the high PLR group (56.0% and 79.5%) (P < 0.05). Kaplan-Meier analysis demonstrated that the RFS in the low PLR group was 45.2% which was significantly higher than that of the high PLR group (20.5%) (X2 = 24.019, P < 0.05). This result suggested that preoperative PLR is a predictor for recurrence followed thermal ablation in RHCC patients, and patients with PLR ≥ 87.87 indicated higher RFS, which may improve the clinical management of RHCC patients. Further studies are warranted to validated this finding and test its clinical applicability in RHCC.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide, which typically arises from a background of cirrhosis, most commonly caused by hepatitis B/C, alcoholism, exposure to food contamination by mycotoxins, or to obesity [Citation1,Citation2]. HCC develops in a multistage process involving chronic liver injury and local inflammation, progressive liver fibrosis and cirrhosis, initiation of neoplastic niches, and malignant transformation. There are various methods to treat HCC, including liver transplantation, partial hepatectomy, percutaneous thermal ablation therapy and transarterial chemoembolisation (TACE). Although the refinements of these treatments have advanced, the prognosis of HCC remains unsatisfactory. The main reason is the high incidence of recurrence of HCC after treatment [Citation3,Citation4]. These facts highlight the importance of risk prediction, active surveillance, and early diagnosis in HCC management.
Lymphocytes are the main component of immunity cells and play a vital anti-tumour role. Lymphocytes are considered a surrogate marker for the immune status of cancer patients and a prognostic factor for recurrence and survival in several cancers [Citation5,Citation6]. To some extent, lymphocytes serve as a useful barometer of immune function and general health in humans [Citation7]. Cancer patients frequently show decreased lymphocytes at diagnosis. In 1970 Riesco reported that lymphocytes were positively associated with the ‘curability’ of a variety of cancers [Citation8]. Similar associations between lymphocytes and survival have been reported for a wide variety of epithelial and connective tissues and lymphoid cancers [Citation9] including HCC [Citation6,Citation10]. The liver itself is an immunity organ, and the immune system strongly influences outcome in patients with HCC. Some reports have suggested that platelet-to-lymphocyte ratio (PLR) is a significant prognostic indicator in some solid cancers [Citation11,Citation12]. This indicator, based on readily available and inexpensive tests, could potentially be an ideal biomarker of outcome in RHCC patients.
Platelets, one of the components in peripheral blood, are simple, easy and cheap to measure. After being first reported by Armand Trousseau, several studies have shown that platelets might play a pivotal role in the development, progression, and angiogenesis as well as metastasis of cancer. Several types of cancer with advanced stages manifested increased platelet counts, and patients with thrombocytosis usually presented poorer prognosis [Citation13–16]. After initial reports that serum vascular endothelial growth factor (VEGF) was increased in cancer patients, as published in 1997, serum VEGF concentrations in breast cancer patients were determined by platelet counts and not by tumour burden [Citation17]. It was reported that platelets release VEGF on activation [Citation18]. In addition, we found evidence that platelets may be involved in tumour-induced angiogenesis because of their release of angiogenic growth factors on activation by angiogenic endothelial cells [Citation19]. Apart from VEGF, platelets contain several other angiogenic growth factors and inhibitors that are released on activation, including platelet-derived endothelial cell growth factor (PD-ECGF), transforming growth factor (TGF), hepatocyte growth factor (HGF), thrombospondin, and even endostatin [Citation20]. Based on these findings, we hypothesised that PLR might be a predictor for recurrence risk in RHCC patients following thermal ablation. To the best of our knowledge, there has been no report evaluating PLR as a risk predictor.
Materials and methods
Patients
Between January 2010 and May 2014, 414 RHCC patients (329 male and 85 female, mean age 59.5 ± 12.1 years (range 28–82 years)) with 960 HCC nodules detected on contrast enhanced US/CT/MRI were enrolled in this study. All patients underwent thermal ablation with curative intention in our institution, and the protocol was programmed in accordance with the patients’ medical history, CT/MRI imaging and laboratory examination. The maximum diameter of lesions ranged from 1.0–6.0 cm (mean ± SD: 2.5 ± 1.2 cm). Curative pre-treatment included liver resection (n = 73), TACE (n = 149) and thermal ablation (n = 192). Types of infection virus were as follows: HBV infected 330, HCV infected 39, HBV + HBV infected 9, and no virus infected 36. Child-Pugh of RHCC patients was evaluated as level A (n = 396) and level B (n = 18). PLR was defined as the ratio of the number of platelets to the number of absolute lymphocytes. Final diagnosis of HCC was verified by pathological examination. This study was approved by the Medical Ethics Committee of Chinese PLA General Hospital. Written informed consent was obtained from every patient.
Pre-ablation examination
The inclusion criteria for our study were as follows: 1) non-resectable tumours or patient refusal to undergo surgery, 2) a single HCC lesion ≤6 cm, 3) two or more] HCC lesions with a maximum diameter ≤4 cm, 4) absence of portal vein thrombosis or extrahepatic metastases, 5) prothrombin time <25 s, 6) prothrombin activity >40%, 7) platelet count >60 cells × 109/L. The exclusion criteria were listed as follows: 1) severe cardiopulmonary disease, 2) serious renal function failure, 3) severe liver function failure, such as large-volume ascites, hepatic encephalopathy, serious oesophageal gastric varices, 4) active severe infection. All patients had undergone conventional ultrasound (US), contrast enhanced US (CEUS), contrast-enhanced CT and/or MRI to delineate the target tumour before ablation.
Microwave ablation protocol
Before ablation, US/CEUS-guided biopsy was performed firstly using an automatic biopsy gun with an 18-gauge cutting needle under local anaesthesia with 1% lidocaine for the RHCC patients without pathological diagnosis at first time treatment. Two or three punctures were performed. Subsequently the antennas were percutaneously inserted into the tumour and placed in the desired location by the guidance of US or CEUS. For lesions <1.7 cm in diameter, a single antenna was used; for those ≥1.7 cm in diameter we used two or more antennas. General anaesthesia (propofol, 6–12 mg/kg/h, ketamine 1–2 mg/kg) was employed, and ablation was implemented. If the lesion was adjacent to the bile duct, gall bladder or bowel (≤5 mm), a 21 gauge thermocouple was placed percutaneously at a designated location to monitor temperature in real time [Citation21]. The temperature was kept at 50–54 °C for no longer than 3 min, with intermittent emission of microwaves for all lesions [Citation22]. If the lesion is near the diaphragmatic dome, artificial ascites should be used. In order to acquire complete ablation for lesions larger than 5 cm or very close to the bile duct, gall bladder or bowel, ethanol (< 2 mL) was applied by injection into the marginal tissue of the tumour through a 21-gauge PTC needle during the ablation. When the hyper-echo overlapped the whole lesion the antennas were withdrawn. During the process, the needle tracks were routinely cauterised to avoid bleeding and tumour seeding.
Follow-up
Complete ablation was defined as no enhancement in any areas of the lesion on enhanced images obtained at 1 month after thermal ablation. The follow-up period started at Feb 2010 and continued until May 2014, ranging from 12–52 months. During this period, the routine contrast-enhanced US and CT and/or MRI were repeated to monitor for recurrence or metastasis at 3 months after ablation and then every 6 months subsequently. Once two consecutive contrast-enhanced images were positive or US-guided core needle biopsy was positive, the follow-up was terminated.
Statistical analysis
Data were analysed using SPSS for Windows (Version 17.0). All data were expressed as the mean ± SD. Preoperative clinical parameters that had impact on recurrence-free survival (RFS) were entered into the multivariate Cox proportional hazard model to determine their independent effect. The best cut-off value of preoperative PLR was determined by time-dependent receiver operating characteristic (ROC) curve analysis [Citation23]. Independent χ2 tests were used to compare categorical variables. Continuous variables were compared using unpaired ttests. RFS curves were analysed using the Kaplan-Meier method and compared using the log-rank test. P < 0.05 was considered significant statistical difference.
Results
Correlation between clinical parameters and RHCC recurrence followed thermal ablation
To determine the clinical parameters correlated with recurrence after thermal ablation in RHCC patients, we performed multivariate Cox proportional hazard model analysis. The statistically significant predictive factors for recurrence identified are shown in . Among the 15 clinical parameters (pre-treatment, age, gender, tumour number, size of tumour, type of hepatitis, cirrhosis, tumour differentiation, PT, alpha-fetoprotein (AFP), PLR, ALC, platelet count (PLT) and Child-Pugh), these results found that poorly differentiated, low PT, low ALC and high PLR were considered as predictive factors for cancer recurrence in RHCC patients.
Table 1. Multivariate Cox regression analysis of factors in relation to recurrence-free survival of RHCC patients.
Selection of the best cut-off value of PLR for recurrence in RHCC patients
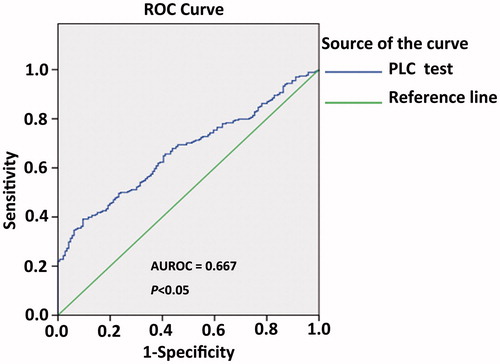
To analyse the predictive value of PLR for recurrence in RHCC patients following thermal ablation, time-dependent ROC curve was performed. A PLR of 87.87 was the best cut-off point for predicting recurrence after thermal ablation in RHCC patients (AUROC was 0.667, P < 0.05) (). We therefore utilised the PLR cut-off of 87.87 as a risk factor for RHCC recurrence. All patients were divided into two groups: a low (< 87.87) PLR group (n = 248, 59.9%) and a high (≥ 87.87) PLR group (n = 166, 40.1%). This result further indicates that the majority of RHCC patients are inclined to recurrence and metastasis after thermal ablation.
Comparisons of recurrence rate in low and high PLR groups of RHCC patients
During the follow-up period, the 1- and 3-year recurrence rates were 39.9% and 54.8% in low PLR group, respectively, which were better significantly than those of high PLR group (56.0% and 79.5%, P < 0.05); 95% is the confidence interval of the indicated recurrence rates in both groups. The recurrence-free overall median survival rates were 13 and 9 months in low and high PLR groups, respectively. Therefore, PLR is an independent risk factor for the risk of cancer recurrence in RHCC patients following thermal ablation.
Comparison of the recurrence-free survival in low and high PLR groups of RHCC patients
To compare the difference of RFS rate between the low PLR and high PLR groups of RHCC patients, the difference of basic clinical parameters between the two groups was first eliminated. The 14 clinical parameters of the low ALC and high ALC groups are compared in and . Among the results, the tumour size, ALC, PLT and AFP were detected as significantly different between the two groups, but the indexes (tumour size, PLT and AFP) were not independent predictive factors for cancer recurrence in the Cox proportional hazard model analysis. There was a significant difference in ALC between the two groups, but ALC was an index of PLR. So the difference maybe acceptable. The RFS rate after thermal ablation was 45.2% in the low PLR group and 20.5% in the high PLR group (P < 0.05). The RFS rates of patients in the low and high PLR groups are shown in . The RFS rate was significantly higher in the low PLR group than the high PLR group (P < 0.05).
Figure 2. Comparison of recurrence-free survival rates (RFS) in the low (< 87.87) and high (≥87.87) PLR groups. The recurrence-free survival rate was significantly higher in the low PLR group than that in the high PLR group (P < 0.05). 59 × 39 mm (300 × 300 DPI).
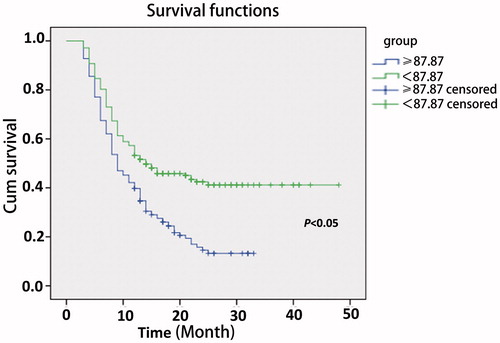
Table 2. Comparison of clinical parameter of RHCC patients between the low and high PLR groups.
Table 3. Comparison of clinical parameters of RHCC patients between the low and high PLR groups.
Discussion
The ultimate goal of treatment for HCC is to prolong survival by eradicating malignant lesions while preserving hepatic function, and to promote the status of immunity to improve the quality of life. HCC develops in a background of chronic hepatitis and inflammation, and in most patients is accompanied by hypersplenism. Thermal ablation, as a technical ease, safety, cost-effectiveness and minimal invasiveness therapy, is the most widely used method in clinics. Some patients with poorer hepatic function and low platelet count can endure the treatment. To eliminate the effect of hypersplenism, the inclusion criteria for platelets was > 60 cells × 109/L in the study which is higher than the suggested guideline [Citation24]. To some extent, the effect of thrombocytopenia on HCC patients may be excluded. Lymphocytes are important immunity cells in HCC patients. Therefore platelets and lymphocytes may play a vital role in HCC patients following treatment.
There have been several studies of the interaction among platelets, lymphocytes and tumour biology for several human cancers [Citation25–27], and PLR is a good predictor for the risk of post-liver transplantation recurrence and may represent an additional tool to refine the selection criteria of HCC liver recipients. As to RHCC patients, the predictive value of PLR has not been reported yet.
In this study, multivariate Cox proportional hazard model analysis showed that PLR is an independent risk factor for re-recurrence in RHCC patients following thermal ablation. The conclusion drawn from this study was similar to that in HCC treated by liver transplantation [Citation27]. HCC patients have mostly lost the chance to receive liver transplantation when they are diagnosed as HCC. These patients are more inclined to accept thermal ablation. In this way, to investigate the value of PLR in predictive recurrence for RHCC patients is very meaningful.
The best cut-off point of PLR for predicting recurrence after thermal ablation in RHCC patients was determined as 87.87 with a relatively high sensitivity and specificity. The difference of independent factors between the high and low groups was eliminated first. The 1- and 3-year recurrence rates and recurrence-free overall median survival were compared, the low group with better results than the high one. As to RFS rate in the follow-up period, the consistent conclusion that low preoperative PLR indicated higher RFS rate in RHCC patients after thermal ablation.
Several mechanisms have been proposed for involvement of platelets in cancer, including promoting cell proliferation, altering cell adhesion, enhancing coagulation and platelet-derived inflammatory cytokines, angiogenesis factors and/or tumour growth factors. Thus, platelet changes can occur in conjunction with coagulation changes in response to the growth of tumours, and conversely platelets may be involved in tumour growth and metastasis [Citation28,Citation29]. In HCC, platelet factors that might be involved in tumour growth include inflammatory cytokines, VEGF, fibroblast growth factor (FGF), serotonin, and platelet-derived growth factors (PDGF), Insulin-like growth factor-1 (IGF-1), epidermal growth factor (EGF), transforming growth factor beta (TGF-β), PDGF, FGF, VEGF, serotonin and interleukins [Citation30]. So platelets may be a risk predictor in HCC. As the results of this study show, PLR was analysed as an independent factor for recurrence in RHCC patients. In some ways platelets may affect the HCC recurrence and metastasis following treatment. Lymphopaenia represents the loss of an anti-tumour specific immune response and the level of immunosuppression. The human body is a balance of immune status. Once the balance is disrupted a tumour may occur and progress [Citation31,Citation32]. A reduction of lymphocytes may predict an incline to recurrence and poor prognosis of cancer patients. So in this study we analysed the value of PLR to predict the recurrence and prognosis in RHCC patients after thermal ablation, and explore the interaction of the two items (platelets and lymphocytes). As reported in colorectal cancer, the levels of IL8, GM-CSF and VEGF levels were higher in tumours, and the cytokine levels were correlated with low lymphocyte levels and high TNM stage. This phenomenon indicates that the elevation of IL8, GM-CSF and VEGF levels in tumours reflects a feature of advanced disease and poor prognosis [Citation33]. A low level of lymphocytes induces a high level VEGF, which is related to cancer recurrence and poor prognosis. So the platelets and lymphocytes are related to VEGF. This is similar to the results in our study. A high ratio of platelets to lymphocytes predicts stronger inclination to recurrence and poor prognosis in RHCC patients after thermal ablation.
The conclusions drawn from this study are limited by the retrospective nature of the analysis and the relatively small number of patients enrolled in the one centre, and the mechanisms of the interaction of PLR and recurrence and poor prognosis in RHCC patients were not explored. Several other studies have reported that larger HCC tumour size is correlated with worse outcomes, and it seems that the tumour size is an important risk factor in HCC treatment [Citation34,Citation35]. But the same conclusion was not drawn in this study. This could be a limiting factor. However, our study is also limited by the larger tumour sizes in the high PLR test group as related to controls. While our study did not demonstrate tumour size to be an independent risk factor for recurrence, multiple other studies have shown that to be the case. It is possible that the larger tumour sizes also play a role in increased rate of recurrence in the high PLR group. Therefore, additional multicentre prospective studies are needed to confirm and advance the findings demonstrated in this study.
Conclusion
In conclusion, this study suggests that RHCC patients with high preoperative PLR are more inclined to recurrence and poorer prognosis after thermal ablation. The best cut-off value for predicting recurrence is 87.87, and this value may benefit the clinical management of RHCC patients before and after thermal ablation. Furthermore, PLC is a simple, easily detected, costless item in the clinic. The powerful prognostic value may benefit most RHCC patients. Further studies are warranted to validated this finding and test its clinical applicability in RHCC.
Conflicts of interest
This work was supported by two grants, numbers 81071210 and 81127006, from the National Scientific Foundation Committee of China. The authors alone are responsible for the content and writing of the paper.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90
- Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, et al. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology 1993;18:1121–6
- Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology 2000;32:1224–9
- O’Keefe SJ, El-Zayadi AR, Carraher TE, Davis M, Williams R. Malnutrition and immuno-incompetence in patients with liver disease. Lancet 1980(8195pt1);2:615–17
- Nagai S, Abouljoud MS, Kazimi M, Brown KA, Moonka D, Yoshida A. Peritransplant lymphopenia is a novel prognostic factor in recurrence of hepatocellular carcinoma after liver transplantation. Transplantation 2014;97:694–701
- Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012;10:33
- Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer 1970;25:135–40
- Ray-Coquard I, Cropet C, Van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 2009; 69:5383–91
- Li X, Han Z, Cheng Z, Yu J, Yu X, Liang P. Prognostic value of preoperative absolute lymphocyte count in recurrent hepatocellular carcinoma following thermal ablation: a retrospective analysis. Onco Targets Ther 2014;7:1829–35
- Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg 2010;200:197–203
- Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Choi HJ, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216–22
- Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J 1996;9:1826–30
- Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol 2002;9:287–91
- Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499–503
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123–34
- Verheul HM, Hoekman K, Luykx-de Bakker S, Eekman CA, Folman CC, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res 1997;3:2187–90
- Verheul HM, Pinedo HM. The importance of platelet counts and their contents in cancer. Clin Cancer Res 2003;9:3219–21
- Verheul HM, Jorna AS, Hoekman K, Broxterman HJ, Gebbink MF, Pinedo HM. Vascular endothelial growth factor-stimulated endothelial cells promote adhesion and activation of platelets. Blood 2000;96:4216–21
- Ma L, Elliott SN, Cirino G, Buret A, Ignarro LJ, Wallace JL. Platelets modulate gastric ulcer healing: role of endostatin and vascular endothelial growth factor release. Proc Natl Acad Sci USA 2001;98:6470–5
- Huang H, Liang P, Yu XL, Cheng ZG, Han ZY, Yu J, et al. Safety assessment and therapeutic efficacy of percutaneous microwave ablation therapy combined with percutaneous ethanol injection for hepatocellular carcinoma adjacent to the gallbladder. Int J Hyperthermia 2015;31:40–7
- Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation – complications among cohort of 1136 patients. Radiology 2009;251:933–40
- Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–44
- Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 2013;19:5430–8
- Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther 2008;8:1247–55
- Carr BI, Guerra V, Pancoska P. Thrombocytopenia in relation to tumor size in patients with hepatocellular carcinoma. Oncology 2012;83:339–45
- Tanoglu A, Karagoz E. Neutrophil and platelet-to-lymphocyte ratio: new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer? Transpl Int 2014;27:e80–1
- Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, et al. Platelet-derived serotonin mediates liver regeneration. Science 2006;312:104–7
- Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med 2013;24:393–400
- Carr BI, Cavallini A, D’Alessandro R, Refolo MG, Lippolis C, Mazzocca A, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer 2014;14:43
- Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol 2014;20:1657–66
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013;39:11–26
- Kim YW, Kim SK, Kim CS, Kim IY, Cho MY, Kim NK. Association of serum and intratumoral cytokine profiles with tumor stage and neutrophil lymphocyte ratio in colorectal cancer. Anticancer Res 2014;34:3481–7
- Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 2013;19:5430–8
- Chan AC, Fan ST, Poon RT, Cheung TT, Chok KS, Chan SC, et al. Evaluation of the seventh edition of the American Joint Committee on Cancer tumour-node-metastasis (TNM) staging system for patients undergoing curative resection of hepatocellular carcinoma: implications for the development of a refined staging system. HPB (Oxford) 2013;15:439–48