Abstract
Purpose: To explore the reliability and validity of radiofrequency (RF) ablation in treating uterine fibroids. Materials and methods: We evaluated 63 patients who underwent hysterectomy to treat multiple fibroids. Thirty patients immediately underwent abdominal hysterectomy after the fibroids were ablated under direct vision. Thirty-three patients first experienced trans-vaginal ablation with the guidance of a baseline ultrasound. We performed abdominal or trans-vaginal hysterectomy 72 h later. The tissues in the centre of the ablated lesion (group A), at the edge of the ablated lesion (group B), 1 cm away from the ablated edge (group C) and the control group were sampled. We observed ultra-structure changes by transmission electron microscopy and detected survivin expression with Western blot analysis. Results: According to transmission electron microscopy, the ultra-structure of fibroid cells in groups A and B was damaged. However, in group C, the ultra-structure was normal. Compared with the control group, survivin expression was significantly decreased. Meanwhile survivin expression was significantly increased with the distance to the ablated centre (p < 0.05). Conclusions: Radiofrequency ablation caused permanent and irreversible damage to fibroid cells and decreased survivin expression, which provided reliable clinical evidence for the success of radiofrequency ablation treating uterine fibroids.
Introduction
Radiofrequency (RF) ablation makes use of a high frequency alternating current to produce biological heating to 85–100 °C, which in turn causes coagulative necrosis in the target tissue and in situ inactivation of the diseased tissue [Citation1]. RF ablation is widely used to treat many solid tumours. The use of RF ablation to treat uterine fibroids has the advantage of confirmed efficacy, less trauma and easy accessibility [Citation2–4]. Although the histology of fibroid tissue has been investigated after RF ablation [Citation5], no-one has as yet looked at ultra-structure and survivin gene expression. Survivin is an apoptosis inhibition protein, which has been demonstrated to be a promising prognostic indicator, and is associated with worse overall survival in a number of cancers, including carcinomas of breast, lung and ovary [Citation6–8]. However, its role in fibroids has rarely been reported. In this study we evaluated changes in the uterine fibroid ultra-structure and survivin gene expression to thoroughly investigate the mechanism of RF ablation in treating fibroids.
Materials and methods
Clinical data
The local institutional ethics committee approved this study, and informed consent was obtained. We evaluated 63 patients who needed to undergo hysterectomy or myomectomy for multiple uterine fibroids, as verified by ultrasound. The patients’ ages in the two groups ranged from 25 years to 55 years, and the average age was 42.4 ± 4.5 years. None of the patients had complications from other pelvic diseases, serious medical diseases or were taking other therapeutic measures in the last 3 months. All operations were conducted in the 14 days before expected menstruation. Thirty patients underwent RF ablation under direct vision after laparotomy; then, hysterectomy was immediately performed, and these patients formed the acute group. The remaining 33 patients first underwent ultrasound-guided trans-vaginal ablation; 72 h later we performed abdominal or trans-vaginal hysterectomy, and these patients were labelled the chronic group. All of the specimens were confirmed uterine fibroids that lacked myoma degeneration by post-operation pathological examination. The endometrium appeared to have a proliferative phase change according to postoperative pathology.
Apparatus
The BBT RF type B multi-functional RF therapeutic apparatus was manufactured by BanBianTian Medical Equipment in Xian, China. The operating frequency was 550 ± 50 KHz, The output power of the generator was 0–60 W. When treating uterine fibroids, the apparatus’s output power was 30 W, the average working time of the apparatus was 5 ± 2 min. A CUB-315 baseline ultrasound apparatus was used for diagnosis and treatment, which was provided by Hitachi (Tokyo, Japan). The frequency of the probe was 3.5 MHz. A transmission electron microscope was supplied by Japan RiLi, Hong Kong.
Treatment methods
Acute experiment group
Thirty patients underwent continuous epidural anaesthesia. After the uterus and fibroids were exposed, we chose to ablate a subserous fibroid or intramural fibroid that was protruding through the serosal surface. The diameter of those fibroids ranged from 3–6 cm, the average diameter was 5 ± 0.5 cm, and the average number of fibroids was three. According to the fibroid size and shape, we selected an appropriate cutting head, which we inserted into the fibroid and then switched on. After the RF ablation apparatus stopped working, we resected the uterus or removed the fibroids.
Chronic experiment group
Thirty-three patients underwent routine vagina cleaning for 3 days. Diclofenac sodium suppositories were adopted through anal pre-operation, and, when necessary, 50 mg of dolantin was injected through the muscle. The patients’ bladders were fully filled. We ablated a subserous or intramural fibroid protruding through the serosal surface with ultrasound guidance. The diameter of the fibroids ranged from 3–6 cm, the average diameter being 5 ± 0.5 cm. The average number of fibroids was two. Abdominal or trans-vaginal hysterectomy was performed 72 h later.
Specimen preparation
The ablated fibroid was cut along the chosen direction with a RF knife. We defined the centre of the ablated lesion as group A, the edge of the ablated lesion as group B, and 1 cm away from the ablated centre as group C. Non-ablated fibroids in the same uterus were assigned to the control group.
Western blot analysis for survivin protein expression
Western blot analysis was performed according to standard methods. Tissue lysates were separated in 12% sodium dodecyl sulphate polyacrylamide gels with 30 μg of protein from each sample loaded per lane. Polyvinylidene difluoride membranes (Millipore, USA) were incubated overnight at 4 °C with primary polyclonal survivin antibody (diluted 1:1000, Cell Signaling Technology, Danvers, MA, USA); then they were incubated with a peroxidase-conjugated secondary antibody (Santa Cruz Biotech, Santa Cruz, USA, diluted 1:2000) for 1 h at room temperature. The immunodetection was performed according to the chemiluminescence protocol (Pierce, Rockford, IL). The image was analysed using Quantity One software (Bio-Rad, Hercules, CA), and GAPDH was used as an internal control.
Statistical analysis
All statistical analyses were performed with SPSS19.0 (SPSS Inc., Chicago, IL, USA). Comparisons among all groups were performed with a one-way analysis of variance (ANOVA) test. If statistical significance was found, Tukey's post hoc test was used. Values of p < 0.05 were considered statistically significant.
Ethics approval
This study was approved by the JiNan University ethics committee, 23 August 2013, reference number IEC-ZN.01-04.3.
Results
The ultra-structure changes of fibroids after RF ablation
In the acute experiment group, hysterectomy was performed immediately after ablation. Electron microscopy showed fibroid cells in group A had muscle contraction, dissolution, fracture and nuclear content outflow, and completely damaged ultra-structure. In group B fibroid cells were damaged, vascular endothelial cells had proliferated and fallen off, thrombosis had formed within the lumen, interstitial vasculature was occluded and inflammatory cells had infiltrated. In group C the fibroid tissue had slightly vacuolated mitochondria, normal nuclei and chromosomes were present, and inflammatory cell infiltration was observed ().
Figure 1. The ultra-structure changes of a fibroid cell immediately after RF ablation. (a) In the centre of the ablated lesion the fibroid cell had muscle contraction, dissolution, fracture and nuclear content outflows. (b) At the edge of the ablated lesion the vascular endothelial cell had proliferated, thrombosis had formed within the lumen, the interstitial vascular was occluded and inflammatory cells had infiltrated. (c) At 1 cm away from the center of the ablated lesion, the fibroid tissue had slightly vacuolated mitochondria, a normal nucleus and chromosomes, and inflammatory cell infiltration.
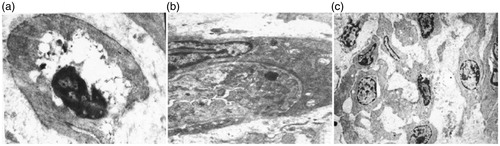
In the chronic experiment group, abdominal or trans-vaginal hysterectomy was performed 72 h after RF ablation. According to electron microscopy, in group A the structure of the fibroid cell was damaged and disappeared, the cytoplasm was vacuolated, and cell nuclei were hyperchromatic and pyknotic. In group B the membrane of the fibroid cell was ruptured, cytoplasm had overflowed, chromatin was condensed, and the nucleoplasm was disproportionate. Additionally, the vascular endothelial cell proliferated, and the interstitial vasculature was occluded in the necrotic zone. In group C, the structure of fibroid was normal, and many new vessels and inflammatory cells were visible, while the fibroblasts proliferated ().
Figure 2. The ultra-structure changes of the fibroid cell 72 h after RF ablation. (a) At the centre of the ablated lesion the cytoplasm was vacuolated and cell nucleus was hyperchromatic and pyknotic. (b) At the edge of the ablated lesion one fibroid cell was ruptured, the cytoplasm had overflowed, the chromatin was condensed and the nucleoplasm was disproportionate. The vascular endothelial cell had proliferated and interstitial vasculature was occluded in the necrotic zone. (c) At 1 cm away from the centre of the ablated lesion, the structure of the fibroid cell was generally normal, many new vessels and inflammatory cells were visible, and the fibroblasts had proliferated.
The expression of survivin 72 h after RF ablation
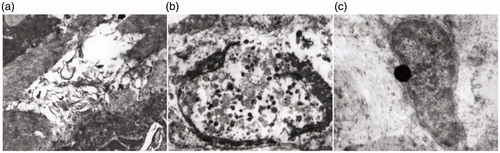
As shown in , the expression of survivin was mostly decreased in group A, which was 4.23 ± 1.22. The survivin expression levels in groups B and C and the control group were19.74 ± 6.22, 32.03 ± 8.01 and 44.89 ± 6.49 respectively. The survivin expression levels in groups A, B and C were significantly lower than the control group (p < 0.05).
FIGURE 3. The expression of survivin in the fibroid cell 72 h after RF ablation. (a) In the centre of the ablated lesion, the survivin expression was weakened. (b) At the edge of the ablated lesion, the survivin expression was positive, at a level of 19.74 ± 6.22. (c) At 1 cm away from the centre of the ablated lesion, the survivin expression was positive, at a level of 32.03 ± 8.01. (d) In the control group, the survivin expression was rarely damaged.

Discussion
The effectiveness of RF ablation for treating uterine fibroids
RF ablation uses a high frequency alternating current to produce biological heat, which causes tumour cell DNA damage and cell death, which was different from other modalities of thermal ablation of fibroids such as microwaves, electroporation methods [Citation9] and high intensity focused ultrasound ablation [Citation10]. RF ablation can effectively kill local tumour cells, and it has been successfully applied to treat liver [Citation11], kidney and prostate tumours as well as cervical intraepithelial neoplasia, dysfunctional uterine bleeding [Citation12], and gynaecological tumours. Recently, studies on the use of RF ablation to treat uterine fibroids have emerged. Jones et al. [Citation13] reported that RF ablation causes rapid solidification and necrosis of the ablated fibroid. Luo et al. [Citation5] reported that RF ablation results in irreversible necrosis in fibroid cells and decreases ER/PR expression. Many studies have demonstrated that RF ablation treatment of uterine fibroids has the advantage of a good curative effect, less trauma and high safety [Citation14].
The ultra-structure changes of uterine fibroids after RF ablation
Investigators have studied the effect of RF ablation on in vitro uterine fibroids and observed a change in the structure, including irreversible necrosis of the fibroid tissue [Citation13]. In our study we performed RF ablation on in vivo fibroids to observe the change in the tissue structure after RF ablation. In the acute experiment group, the ultra-structure of fibroid cells in group A appeared completely damaged. In group B the ultra-structure of the fibroid cells was damaged, and the vessel endothelial cells had proliferated. In group C the cell structure had mild vacuoles in the mitochondria. The results in the acute experiment were similar to those in the chronic experiment group. At 72 h after RF ablation the ultra-structure of the fibroid cell in groups B and C did not appear recovered. Outside of the ablated lesion, there were more new blood vessels; meanwhile, granulation tissue and fibroblasts had proliferated. Additionally, inflammatory cells had infiltrated. All of those results verified that there were irreversible changes in the ultra-structure after RF ablation, which further confirmed the safety and efficacy of RF ablation in treating uterine fibroids.
The effect of RF ablation on the expression of survivin
Survivin belongs to the family of apoptosis inhibition proteins, and it inhibits cell apoptosis, protects tumour blood vessels and promotes cell proliferation [Citation15]. Many studies have shown that survivin has an intimate relationship with cervical cancer [Citation16], endometrial carcinoma [Citation17], ovarian cancer [Citation18], trophoblastic tumours [Citation19] and endometriosis [Citation20]. However, the relationship between survivin and uterine fibroids has rarely been reported. In the chronic experiment group in our study, the centre of the ablated lesion lacked survivin expression, but there was survivin expression in the unablated fibroid tissue, as well as in groups B and C. Furthermore, the survivin expression in groups A, B and C was significantly lower than that in the unablated fibroid tissue. The expression of survivin was weakened in the part of the lesion that was close to the RF region of the cutting head, where the local temperature was higher. Therefore, the heat energy produced by RF ablation could have effectively decreased the expression of the survivin gene and alleviated the effect of survivin on inhibiting apoptosis, which could induce fibroid cell apoptosis and contribute to killing tumour cells.
Conclusions
RF ablation induced an irreversible change in the fibroid cell ultra-structure and decreased the survivin expression. We speculated that the cell apoptosis induced by RF ablation might be one of the mechanisms by which this technology effectively treats uterine fibroids. RF ablation has little effect on the normal muscle tissue around the fibroid, making it a safe and effective approach for uterine fibroid treatment.
Acknowledgements
We thank LetPub for linguistic assistance during the preparation of this manuscript.
Declaration of interest
This work was supported by the Cultivating Innovation Fund of Ji Nan University (11614305). The authors alone are responsible for the content and writing of the paper.
References
- Lopez-Molina JA, Rivera MJ, Trujillo M, Burdio F, Lequerica JL, Hornero F, et al. Assessment of hyperbolic heat transfer equation in theoretical modeling for radiofrequency heating techniques. Open Biomed Eng J 2008;2:22–7
- Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: Radio–frequency ablation of medium and large lesions. Radiology 2000;214:761–8
- Iversen H, Lenz S, Dueholm M. Ultrasound-guided radiofrequency ablation of symptomatic uterine fibroids: Short-term evaluation of effect of treatment on quality of life and symptom severity. Ultrasound Obstet Gynecol 2012;40:445–51
- Quinn SD, Gedroyc WM. Thermal ablative treatment of uterine fibroids. Int J Hyperthermia 2015;31:272–9
- Luo X, Shen Y, Song WX, Chen PW, Xie XM, Wang XY. Pathologic evaluation of uterine leiomyoma treated with radiofrequency ablation. Int J Gynaecol Obstet 2007;99:9–13
- Li Y, Ma X, Wu X, Liu X, Liu L. Prognostic significance of survivin in breast cancer: Meta-analysis. Breast J 2014;20:514–24
- Zhang LQ, Wang J, Jiang F, Xu L, Liu FY, Yin R. Prognostic value of survivin in patients with non-small cell lung carcinoma: A systematic review with meta-analysis. PLoS One 2012;7:e34100
- Gasowska-Bodnar A, Bodnar L, Dabek A, Cichowicz M, Jerzak M, Cierniak S, et al. Survivin expression as a prognostic factor in patients with epithelial ovarian cancer or primary peritoneal cancer treated with neoadjuvant chemotherapy. Int J Gynecol Cancer 2014;24:687–96
- Seror O. Ablative therapies: Advantages and disadvantages of radiofrequency, cryotherapy, microwave and electroporation methods, or how to choose the right method for an individual patient? Diagn Interv Imaging 2015;96:617–24
- Xie B, Zhang C, Xiong C, He J, Huang G, Zhang L. High intensity focused ultrasound ablation for submucosal fibroids: A comparison between type I and type II. Int J Hyperthermia 2015;31:593–9
- Yin G, Zhu T, Li J, Chen M, Yang S, Zhao X. Decreased expression of survivin, estrogen and progesterone receptors in endometrial tissues after radiofrequency treatment of dysfunctional uterine bleeding. World J Surg Oncol 2012;10:100
- Yin G, Chen M, Yang S, Li J, Zhu T, Zhao X. Treatment of uterine myomas by radiofrequency thermal ablation: A 10-year retrospective cohort study. Reprod Sci 2014;22:609–14
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int 2012;2012:194839
- Oeda S, Mizuta T, Isoda H, Kuwashiro T, Iwane S, Takahashi H, et al. Survival advantage of radiofrequency ablation for hepatocellular carcinoma: Comparison with ethanol injection. Hepatogastroenterology 2013;60(126):1399–404
- Kim YS, Seol CH, Jung JW, Oh SJ, Hwang KE, Kim HJ, et al. Synergistic effect of sulindac and simvastatin on apoptosis in lung cancer A549 cells through AKT-dependent downregulation of survivin. Cancer Res Treat 2015;47:90–100
- Cao XQ, Lu HS, Zhang L, Chen LL, Gan MF. MEKK3 and survivin expression in cervical cancer: Association with clinicopathological factors and prognosis. Asian Pac J Cancer Prev 2014;15:5271–6
- Brunner A, Riss P, Heinze G, Brustmann H. pHH3 and survivin are co-expressed in high-risk endometrial cancer and are prognostic relevant. Br J Cancer 2012;107:84–90
- Kusku CF, Yigit S, Demir L, Cakalagaoglu F, Tarhan O. Correlation of survivin and MMP9 expressions with prognosis and clinicopathological parameters in surface epithelial ovarian carcinomas. Turk Patoloji Derg 2014;30:30–7
- Fest S, Brachwitz N, Schumacher A, Zenclussen ML, Khan F, Wafula PO, et al. Supporting the hypothesis of pregnancy as a tumor: Survivin is upregulated in normal pregnant mice and participates in human trophoblast proliferation. Am J Reprod Immunol 2008;59:75–83
- Lamp M, Saare M, Kadastik U, Karro H, Salumets A, Uibo R, et al. Survivin promoter polymorphisms and autoantibodies in endometriosis. J Reprod Immunol 2012;96:95–100
