Abstract
In 2011 Hanahan and Weinberg updated their well-established paper ‘The hallmarks of cancer’. The rationale for that review and its predecessor was to produce a conceptual framework for future research in cancer. The original Hallmarks included: cell signalling to enhance tumour cell proliferation, acquisition of ability to evade growth suppressors, developing mechanisms to resist cell death, enabling replicative immortality, initiating angiogenesis and activating processes to enable invasion and metastasis. In the more recent paper, Hanahan and Weinberg added important new features to this composite paradigm. The new features were: (1) altered metabolism, (2) evasion of immune destruction, (3) tumour promoting inflammation, and (4) the cellular microenvironment. These four new features are the main focus of this review. Hanahan and Weinberg did not specifically include the physiological microenvironment which is dominated by hypoxia and acidosis. In this review we will consider these features in addition to the cellular and metabolic components of the microenvironment. The purpose of this review is to present a vision of emerging fields of study in hyperthermia biology over the next decade and beyond. As such, we are focusing our attention on pre-clinical studies, primarily using mice. The application of hyperthermia in human patients has been thoroughly reviewed elsewhere.
Introduction
In 2011 Hanahan and Weinberg updated their well-established paper ‘The hallmarks of cancer’ [Citation1]. The rationale for this review and its predecessor [Citation2] was to produce a conceptual framework for future research in cancer. The original ‘Hallmarks’ included cell signalling to enhance tumour cell proliferation, acquisition of ability to evade growth suppressors, developing mechanisms to resist cell death, enabling replicative immortality, initiating angiogenesis and activating processes to enable invasion and metastasis [Citation2]. In the more recent paper, Hanahan and Weinberg added important new features to this composite paradigm. The new features were 1) altered metabolism, 2) evasion of immune destruction, 3) tumour-promoting inflammation, and 4) the cellular microenvironment. These four new features are the main focus of this review. Hanahan and Weinberg did not specifically include the physiological microenvironment, which is dominated by hypoxia and acidosis. In this review we will consider these features in addition to the cellular and metabolic components of the microenvironment (). The purpose of this review is to present a vision of emerging fields of study in hyperthermia biology over the next decade and beyond. As such, we are focusing our attention on pre-clinical studies, primarily using mice. The application of hyperthermia in human patients has been thoroughly reviewed elsewhere [Citation3,Citation4].
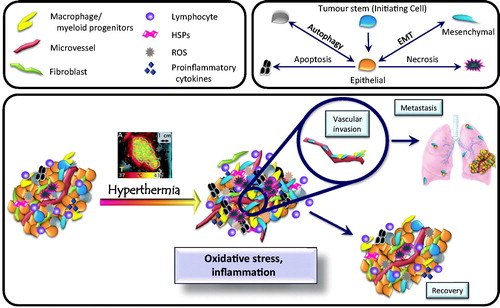
Figure 1. Overview of the tumour microenvironment. The upper boxes contain symbols for cell types found in tumours, along with factors that influence the tumour microenvironment. Many cell types are found, including macrophages, lymphocytes, fibroblasts, and vascular endothelial cells. Cross talk between these cell types promotes tumour growth. The lower box summarises how the microenvironment changes in response to hyperthermia. Under baseline conditions (left), tumours contain a variety of different cell types. Pleiotropic tumour cell phenotypes include stem cells, cells exhibiting epithelial or mesenchymal characteristics, and cells undergoing autophagy. Oxidative stress and inflammation then influence which phenotype predominates following hyperthermia treatment. Changes induced by hyperthermia include (1) altering cytokine and chemokine levels, (2) promoting tumour cell survival, (3) driving epithelial cells toward mesenchymal (invasive) phenotype, (4) promoting metastasis, and (5) altering immunogenicity of the remaining tumour cells.
This review excludes discussion of traditional mechanisms of action of hyperthermia as an adjuvant to radiotherapy or chemotherapy. An excellent review of these historical subjects is available [Citation5] There is minimal discussion of the effects of hyperthermia on nanoparticle transport to tumours or on the subject of thermally mediated drug delivery from nanoparticles. These subjects have been discussed in a number of reviews [Citation6–10].
Methods
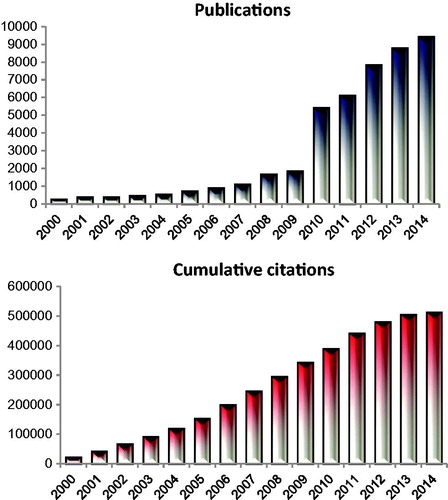
Web of Science™ (Thomson Reuters) was used as a search engine, using the MeSH terms of ‘tumour’ and ‘microenvironment’. This yielded more than 63,000 manuscripts, published since 2000. The numbers of papers per year on these subjects have grown exponentially from fewer than 300 in 2000 to more than 9000 in 2014 (). ‘Tumour’ and ‘microenvironment’ also captured many key papers on tumour metabolism and immune evasion. Astoundingly the collection of papers identified with the key terms ‘tumour’ and ‘microenvironment’ has been cited over 500,000 times! The next step in the search was to determine the links between hyperthermia and tumour microenvironment.
Figure 2. Depiction of the number of publications focusing on the two subjects ‘tumour’ and ‘microenvironment’ since the year 2000. The number of citations has increased to match the citations. The cumulative number of citations focusing on these two MeSH terms is over 500,000. In this review we consider how well the field of hyperthermia has integrated into this emerging hallmark and the opportunities that exist to further the impact of hyperthermia in defining the field of tumour microenvironment.
Additional analyses were performed, cross referencing the hyperthermia terms above with key features of the tumour microenvironment. The key features, identified by searching for the most highly cited papers in the field of ‘tumour microenvironment’ across the continuum of years from 2000–2014 included (1) ‘immunity’ (219 papers), (2) ‘inflammation’ (377 papers), (3) ‘myeloid’ (76 papers), (4) ‘macrophage’ (256 papers), (5) lymphocyte (107 papers), (6) ‘stem cell’ (255 papers), and (7) ‘cellular metabolism’ (3 papers). Clearly there is overlap between some of these MeSH terms. Accordingly, discussion of ‘immunity’, ‘inflammation’, ‘myeloid’ ‘lymphocyte’ and ‘macrophage’ are considered as one theme, below.
A consistent theme in our field has been the emphasis on quantifying hyperthermia treatment parameters, i.e. temperature and time of heating. There is no doubt that the biological effects described below are dependent upon these parameters. To simplify the reporting, however, the studies discussed below will be simply classified as either ‘fever-range total body hyperthermia’ which is typically mild heating to less than 40 °C for 6-h, traditional ‘hyperthermia’, which involves local or regional heating to temperatures between 40 and 45 °C for 30–60 min, or ‘thermal ablation’, which involves reaching tissue temperatures of 60–100 °C for several minutes.
The searches we have described identified hundreds of papers; hence not all can be discussed. We have highlighted some that appear particularly intriguing or insightful. It is hoped that readers of this review will be compelled to search these key words themselves, to identify additional papers that are of interest to them.
Results
Physiological tumour microenvironment: pH
Hyperthermia
A simple cross reference of ‘hyperthermia’ against ‘tumour’ + ‘microenvironment’ yielded 122 hits; the most highly cited papers examined how hyperthermic cytotoxicity is influenced by tumour pH and oxidative stress. Although interest in pH manipulation has waned in recent years, cutting edge research is still being performed. [Citation11–13] Acute intracellular acidification of cells was demonstrated over 20 years ago to sensitise tumour cells to killing by hyperthermia. Leeper and Glickson have continued to pursue the goal of identifying a pharmacological approach that can safely achieve this goal [Citation11]. In particular, they have investigated the use of lonidamine as an agent to create intracellular acidification. This drug was originally developed as an anti-spermatic male contraceptive, but later was found to exhibit anti-tumour effects. It is a potent inhibitor of the lactic acid transporter MCT-1 and the mitochondrial pyruvate transporter. This dual mechanism of action inhibits lactate export and depletes ATP [Citation14]. It is a potent sensitiser for heat killing () and also enhances cytotoxicity of drugs such as melphalan and doxorubicin [Citation11,Citation15]. Given the fact that this drug has been studied in humans previously, the potential path to clinical testing is relatively short.
Figure 3. Hyperthermia-mediated cell killing is enhanced by 150 μM lonidamine. Lonidamine was administered beginning 1 h prior to a 2-h hyperthermia treatment. Cells were grown and treated at the indicated extracellular pH (pHe). Hyperthermia increased cell killing at pHe of both 7.3 and 6.7. Lonidamine had a synergistic effect on cell killing in cells grown at pHe 6.7 only. (Data re-plotted from Coss et al. [Citation14]).
![Figure 3. Hyperthermia-mediated cell killing is enhanced by 150 μM lonidamine. Lonidamine was administered beginning 1 h prior to a 2-h hyperthermia treatment. Cells were grown and treated at the indicated extracellular pH (pHe). Hyperthermia increased cell killing at pHe of both 7.3 and 6.7. Lonidamine had a synergistic effect on cell killing in cells grown at pHe 6.7 only. (Data re-plotted from Coss et al. [Citation14]).](/cms/asset/ecaeed3b-bf2e-4e4f-82e3-897b019fd06e/ihyt_a_1091093_f0003_c.jpg)
Future studies in this field, which will enhance translation, should include combinations of drugs with hyperthermia and acidification.
Thermal ablation
There are virtually no papers in which the physiological consequences of thermal ablation have been reported. One team has evaluated effects of thermal ablation on liposomal drug uptake. The effects seen are undoubtedly related to physiological changes induced by thermal ablation. Ahmed et al. examined differences in non-thermosensitive liposomal doxorubicin uptake in thermally ablated tumours [Citation16]. Thermal ablation was shown to significantly increase drug uptake by 3-fold, as compared with liposome alone. Pre-clinically, the peak in enhanced liposomal drug uptake occurred several days after thermal ablation, and the optimal sequence between the two treatments was to administer the liposome after thermal ablation, with no later than 24 h between the two [Citation17]. Clinically, the combination of thermal ablation with doxorubicin containing liposome therapy increased the ablation zone size, compared with thermal ablation alone [Citation18]. The mechanisms underlying the increase in liposome accumulation were not reported.
Future studies in this field should attempt to identify the mechanisms for enhanced drug uptake. Presumably, the mechanisms would involve some change in perfusion, permeability, stromal components and/or interstitial fluid pressure, since these factors are known to influence nanoparticle drug delivery [Citation19]. It is clear that this is an important direction to follow with other nanoparticle preparations.
Fever-range total body hyperthermia
Two key papers published by Repasky’s group demonstrated that fever-range whole body heating increases perfusion and reduces interstitial fluid pressure and hypoxia in several tumour models [Citation20]. Further, these effects improve radiotherapy response and enhance liposomal drug uptake [Citation20,Citation21].
Future studies could examine in more detail what the underlying mechanisms are for the observed phenotypic changes in tumour physiology. Interestingly, we observed that voluntary exercise exerts many of the same types of effects as those reported by the Repasky group [Citation22,Citation23]. Whether or not there are common mechanisms involved would be an interesting and clinically relevant question.
Tumour immunity, inflammation, autoimmunity and oxidative stress
Heat shock proteins
The role that heat shock proteins (HSPs) play in augmenting immune recognition of tumour cells has been driven by many investigators in the field of hyperthermia. The interest in the combination of hyperthermia with immunotherapy led to publication of two special issues in the International Journal of Hyperthermia. The first, in 2002, edited by Elizabeth Repasky and Rolf Issels, was the first special issue published by the International Journal of Hyperthermia. The nine papers in this special issue have been cited more than 380 times. The second special issue, on hyperthermia and tumour immunity, was published in 2013 and covered the role of HSPs in augmenting immunotherapy [Citation24–41].
Hyperthermia
There are multiple benefits from treatment of tumours with local hyperthermia, as is outlined in an excellent review by Toraya-Brown [Citation42]. Much like total body hyperthermia, described below, local hyperthermia can increase dendritic cell antigen presentation by increasing antigen expression by tumour cells and enhancing heat shock protein production. These effects, combined with alterations in cytokine production and endothelial cell adhesion molecule expression, can enhance T-cell activation and immune cell activity within treated tumours.
Future work needs to consider how anti-tumour immunity is altered when hyperthermia is administered with other therapies, since hyperthermia is not used in isolation. A recent publication by Viglianti et al. points to possible abscopal effects of local hyperthermia, when combined with a thermosensitive doxorubicin-containing liposome [Citation43]. In this experimental design two tumours were transplanted into the flanks of mice, but only one was heated. Anti-tumour effects were seen in both tumours. The role that hyperthermic modification of anti-tumour immunity might play in reducing or altering metastasis formation is a key unresolved question.
Thermal ablation
Specific anti-tumour immune effects have been observed in pre-clinical models after thermal ablation with high intensity focused ultrasound, such as resistance to re-challenge, using the MC38 murine colon carcinoma model [Citation44]. Additionally, augmentation of anti-tumour effectiveness against the TRAMP-C murine prostate cancer model has been seen when combining thermal ablation with the immune checkpoint inhibitor against CTLA-4, ipilimumab [Citation45]. Increased immune responsiveness towards hepatocellular carcinoma has been observed in human patients following thermal ablation, although evidence for systemic immunity was not convincingly demonstrated [Citation46]. In a small clinical series in companion dogs with spontaneous cancers, administration of thermal ablation alone appeared to result in long-term local tumour control. Although not proven, the authors speculated about the role that anti-tumour immunity might have played in these results [Citation47]. Two papers focused on how thermal ablation might enhance tumour antigen presentation to dendritic cells, thereby enhancing anti-tumour immunity [Citation48,Citation49]. On the other hand, incomplete thermal ablation has been shown to enhance aggressiveness and metastasis from tumour cells that survive the procedure [Citation50].
Future studies should emphasise the duality of the effects of thermal ablation. Studies that balance these effects may reveal whether the enhanced antitumour immunity can offset the tendency of residual tumour cells to become more aggressive. One could also consider adding chemotherapy to thermal ablation to examine how such combinations might alter immune responsiveness. Given the greatly enhanced drug delivery and anti-tumour effects seen with thermosensitive liposomes [Citation51] or non-thermally sensitive liposomes, [Citation16,Citation17] this would seem to be a prudent line of investigation.
Fever-range total body hyperthermia
Several key studies demonstrated the enhanced tumour immunity that can occur with this form of hyperthermia. There are multiple mechanisms involved, starting with increasing the propensity for cytotoxic T lymphocyte (CTL) and natural killer (NK) cells to successfully adhere to and extravasate through the tumour microvasculature. Additionally, increased tumour perfusion generated by this therapy grants greater access of immune cells to the tumour parenchyma. [Citation21,Citation41] Effects on the pro-inflammatory cytokine, IL-6 are abrogated, turning its function towards enhancing anti-tumour immunity [Citation52]. Sharon Evans’s team has examined the effects of fever-range hyperthermia (39.5 °C q6h) on immune cell trafficking [Citation41,Citation52,Citation53]. Their most recent paper demonstrates that this form of systemic heating enhances IL-6 trans-signalling and is permissive for cytotoxic T cell trafficking across tumour microvessels [Citation52] (). These effects are mediated by IL-6-dependent enhancement of adhesion molecule expression and upregulation of the signal transducing subunit of the IL-6 family receptor gp130 in tumour endothelial cells after systemic heating; an increase in tumour-cell-specific cytotoxicity is observed. Their results support previous work showing that whole body hyperthermia increases IL-1 and IL-6 levels in non-tumour bearing rats [Citation54].
Figure 4. Hyperthermia augments cytotoxic T cell killing when applied in conjunction with a recombinant hyper-IL-6 fusion protein (H-IL-6). H-IL-6 is comprised of IL-6 and s-IL-6R. When combined with hyperthermia, this treatment promotes CD8+ T cell extravasation through the tumour bed, increasing tumour cell death. (Adapted from Fisher et al. [Citation52]).
![Figure 4. Hyperthermia augments cytotoxic T cell killing when applied in conjunction with a recombinant hyper-IL-6 fusion protein (H-IL-6). H-IL-6 is comprised of IL-6 and s-IL-6R. When combined with hyperthermia, this treatment promotes CD8+ T cell extravasation through the tumour bed, increasing tumour cell death. (Adapted from Fisher et al. [Citation52]).](/cms/asset/601c470c-2dd3-486c-82f7-2f8e787b0137/ihyt_a_1091093_f0004_c.jpg)
Future studies should examine whether total body hyperthermia can enhance anti-tumour effects of systemic treatments, such as chemotherapy and whether the enhanced immune function is maintained or even enhanced under these circumstances. The role that HSP110 plays in directing tumour immune responsiveness has made the most translational headway. A vaccine using this HSP is now in a phase I clinical trial (NCT01744171) [Citation55]. Future studies could delve more deeply into how hyperthermia can affect the other well-known and emerging immune checkpoints [Citation56].
Macrophages
Macrophages are extremely important effectors of innate immunity and inflammatory responses in tissue. They play a direct role in protecting against tissue invasion by pathogens and coordinate the recovery of tissue during wound healing. Macrophages perform multiple functions to accomplish these tasks. However, tumours can co-opt key functions of macrophages to promote tumour growth, angiogenesis and metastasis [Citation57]. The switch between the tumour inhibiting vs. promoting effects of macrophages is reflected by their change from an M1 to an M2 phenotype, respectively. We were unable to identify any papers reporting on whether thermal therapies alter phenotypic differences in macrophages.
Future studies should examine whether thermal therapies alter macrophage phenotypes and if they do, whether these changes play a role in tumour and normal tissue responses to therapy.
Neutrophils
Neutrophils are one of the primary mediators of inflammation within tumours, and modulate T cell responses therein [Citation58]. Fever-range whole body hyperthermia increases neutrophil infiltration in syngeneic and xenografted colon tumours [Citation59]. Neutrophil depletion negated hyperthermia-mediated tumour growth delay in both tumour models. Future studies may choose to examine enhanced activation of the complement cascade, as complement components C3a and C5a promote anaphylaxis. We were unable to locate any studies that examined complement activation in tumours following whole body or localised hyperthermia.
Autoimmunity
Fever-range total body hyperthermia can also function as an immune modulator in murine models of autoimmune diseases such as type 1 diabetes [Citation60] and rheumatoid arthritis [Citation61]. Total body hyperthermia can prevent the onset of type 1 diabetes by decreasing autoreactive CTLs infiltration to pancreatic islets and increasing the cytotoxic activity of NK cells. In addition, total body hyperthermia also reduces arthritis disease progression by decreasing macrophage infiltration and pro-inflammatory cytokine production.
Oxidative stress
Tumour responses to thermal and other stresses can be mediated by oxidative stress. Tumours are typically under chronic oxidative stress. Oxidative stress promotes up-regulation of NFkB and HIF-1; downstream targets, such as XIAP (X-linked inhibitor of apoptosis protein), promote treatment resistance [Citation62] and tumour cell survival [Citation63,Citation64]. The extent of oxidative stress is created by complex cellular and perhaps physiological malfunctions. Tumours lack a full complement of anti-oxidant enzymes (e.g. catalase is often down-regulated), [Citation64] which can lead to build up of peroxide. Peroxide, in turn, up-regulates both NFkB [Citation65] and HIF-1 [Citation66]. Increased NO levels can directly inhibit proteosomal degradation of HIF-1, for example, by nitrosylating a crucial cysteine residue in its oxygen-dependent degradation domain [Citation67]. Up-regulation of HIF-1 can contribute to tumour promotion via multiple mechanisms involving induction of angiogenesis, maintenance of glycolytic metabolism, promotion of metastasis and inhibition of apoptosis, to name a few [Citation68]. Macrophages can also play an important role in increasing oxidative stress in tumours. Further, it has been speculated that cycling hypoxia in tumours, a phenomenon typified by chronic instabilities in oxygenation, can lead to hypoxia-reperfusion injury, thereby contributing to oxidative stress by activation of xanthine oxidase [Citation69,Citation70]. The consequences of oxidative stress on tumour cell survival are complex as well. At low concentrations or fluxes of reactive species, oxidative stress can promote tumour cell survival via the mechanisms described above. High levels of oxidative stress can swamp endogenous anti-oxidant defences, denature proteins, inhibit cellular functions and lead to cell death [Citation71].
Several investigators have shown that oxidative stress is increased by heat. A recent and excellent review of this topic can be found in the International Journal of Hyperthermia [Citation72]. Davidson reported that the cytotoxicity of heat toward Saccharomyces cerevisiae is mediated by an increase in oxidative stress. Mutants deficient in key anti-oxidant enzymes such as catalase were more heat sensitive than wild-type cells [Citation73]. Mitchell and Russo reported that hyperthermia increased cellular levels of a primary antioxidant, glutathione. Depletion of glutathione led to sensitization of heat toxicity [Citation74]. Although this approach worked effectively in vitro, attempts to take advantage of glutathione depletion in vivo were not successful [Citation75]. The origin of increases in oxidative stress are complex, but the primary mediators are changes in mitochondrial metabolism [Citation72] and increases in NADPH oxidase activity [Citation76].
Future studies in the field of hyperthermia/thermal ablation could focus on developing adjuvant strategies to further increase oxidative stress in tumours through the addition of therapeutic agents that accomplish this. By overwhelming endogenous oxidative stress mechanisms a new therapeutic strategy may emerge.
Angiogenesis
Hyperthermia
Moon et al. [Citation76] examined the effects of local hyperthermia on HIF-1 and VEGF levels. HIF-1 was upregulated after application of hyperthermia (42 °C q1h) (). The mechanism was related to up-regulation of NADPH oxidase in tumour cells, via the ERK pathway [Citation76]. This observation is consistent with the hypothesis that local hyperthermia alone could promote tumour angiogenesis. Consistent with this hypothesis, addition of antiangiogenic agents to hyperthermia has demonstrated superior anti-tumour effects, compared with either alone [Citation77,Citation78]. In contrast, Roca et al. [Citation79] reported that the antiangiogenic cytokine, plasminogen activator inhibitor-1 (PAI-1) is up-regulated after hyperthermia and that its antiangiogenic effects reduce tumour regrowth after hyperthermia treatment. Interestingly, both VEGF and PAI-1 are HIF-1 target genes [Citation80]. Thus, the consequence of HIF-1 up-regulation is both pro- and anti-angiogenic. The relative balance between the activity of these factors would ultimately dictate the tissue response. This does not negate the possibility of adding anti-angiogenic therapies to hyperthermia, as a means to tip the balance more toward anti-angiogenesis. Nie et al. [Citation77] have demonstrated remarkable anti-tumour and anti-metastatic activity of a VEGFR2 inhibitor, when combined with local hyperthermia. These effects were observed in two highly metastatic mouse tumours – the 4T1 mammary carcinoma and the CT26 colon tumour.
Figure 5. Hyperthermia induced upregulation of HIF-1 in tumours. 4T1 mouse mammary tumours were grown in the flanks of mice. The tumour-bearing leg was heated at 34 °C or 42 °C for 1 h. HIF-1 expression was assessed by a luciferase reporter gene at various time points post-treatment. Luciferase activity was normalised to tumour volume at the time of treatment. Data points represent mean ± sem; n = 4–8/group. *p < 0.01. (Data re-plotted from Moon et al. [Citation76].) HT, hyperthermia.
![Figure 5. Hyperthermia induced upregulation of HIF-1 in tumours. 4T1 mouse mammary tumours were grown in the flanks of mice. The tumour-bearing leg was heated at 34 °C or 42 °C for 1 h. HIF-1 expression was assessed by a luciferase reporter gene at various time points post-treatment. Luciferase activity was normalised to tumour volume at the time of treatment. Data points represent mean ± sem; n = 4–8/group. *p < 0.01. (Data re-plotted from Moon et al. [Citation76].) HT, hyperthermia.](/cms/asset/41d26303-0ea4-43b1-9da0-34d0428781af/ihyt_a_1091093_f0005_c.jpg)
Thermal ablation
It is unclear whether thermal ablation promotes or inhibits angiogenesis. For example, VEGF levels and angiogenesis were reduced following thermal ablation of the VX2 rabbit carcinoma, when transplanted to the liver [Citation81]. In contrast, insufficient thermal ablation of liver lesions led to an increase in HIF-1 and VEGF production followed by promotion of HepG2 tumour regrowth in the local site [Citation82]. Nikfarjam et al. [Citation83] generated a liver metastasis model by injecting tumour cells into the spleen, followed by splenectomy. Liver metastases form from cells that escape the spleen and enter the splenic vein and portal circulation. Nikfarjam observed increased VEGF levels in liver adjacent to metastatic tumour lesions that had been ablated previously with a laser. This was accompanied by a relative increase in grossly metastatic tumour burden in the ablated liver lobe, compared with other lobes where tumours were not ablated. They concluded that incomplete thermal ablation can enhance growth rate of micrometastases.
Total body hyperthermia
It has been reported that application of whole body hyperthermia to rats (40 min with peak temperature of 42 °C for 20 min) leads to a significant increase in VEGF levels and angiogenesis in cardiac tissues [Citation84]. This strategy was considered as a means to increase perfusion in the heart. Sen et al. [Citation21] demonstrated that mild temperature total body hyperthermia increased tumour perfusion, which was accompanied by an increase in the number of CD31 positive cells, as a tissue marker of vascular density. They did not measure VEGF or other pro-angiogenic factor levels, so it is unknown whether the increase in perfusion was mediated by promotion of angiogenesis.
Future studies could focus on better defining the consequences of thermal therapies on tumour angiogenesis and defining where and when to consider adding angiogenesis inhibitors. Much of the focus of the literature has been on VEGF, but there are a number of other pathways that can stimulate angiogenesis. Elucidation of the relative importance of alternative pathways is of importance in moving forward.
Stem cells
Hyperthermia
The discovery that heat inhibits homologous repair of DNA double strand breaks provides a rationale for using heat to create a ‘synthetic lethal’ approach, if combined with inhibitors of poly (ADP-ribose) polymerase (PARP-1). Stem cells are particularly sensitive to this combination [Citation85,Citation86]. Atkinson examined the effects of radiotherapy alone vs. thermoradiotherapy on the stem cell population in murine and human tumour xenografts [Citation87]. Remarkably, in all tumour lines examined, irradiation led to relative increase in putative stem cells, whereas the combination of radiotherapy and 42 °C heating substantially decreased the stem cell population. The underlying mechanism for difference in survival of the stem cell population was related to thermal inhibition of double strand break repair by the stem cells. A separate study showed that hyperthermia potentiates metformin killing of stem cells [Citation88]. Clinical trials are being designed to test PARP-1 inhibitors in combination with hyperthermia and radiotherapy.
Thermal ablation
There were few papers on stem cells and thermal ablation. One paper reported that thermal ablation increases the infiltration of bone marrow-derived stem cells into an ablated region. This effect was deemed to be positive because these stem cells were shown to differentiate into hepatocytes, which then contribute to the regrowth of the damaged liver [Citation89]. There are potential challenges for this approach, however. Myeloid progenitor cells are known to contribute to repair of tumour microvessel damage after radiotherapy and reduce the anti-tumour effects of radiotherapy [Citation90,Citation91]. Similar mechanisms might be invoked after thermal ablation.
Total body hyperthermia
The most important findings here relate to recovery of bone marrow stem cells after chemotherapy or whole body irradiation, as might occur for bone marrow transplant. Capitano et al. [Citation92] reported that mild temperature whole body heating (39.5 °C for 6 h) increased the rate of recovery of haematopoietic stem cells and neutrophils via mechanisms related to IL-17, G-CSF and IL-1. Whether or not total body hyperthermia could add to standard growth factor treatment remains to be tested.
Future studies should emphasise how hyperthermia/thermal ablation and feverrange total body hyperthermia affect both tumour and normal tissue stem cells. Both can contribute to therapeutic responses. It will be necessary to avoid scenarios where normal tissue stem cells contribute to tumour resistance to therapy.
Cellular metabolism
Hyperthermia
Despite the fact that it has been known for over 30 years that hyperthermia affects tumour cell metabolism, there are only a handful of papers that have examined this subject in detail. The first investigator to report metabolic changes from hyperthermia was Christian Streffer. Abstracts showing his interest in this subject date back to 1983 [Citation93]. Tamulevicius and Streffer reported on ATP, glucose and lactate levels in a human tumour xenograft line 1 h after heating at 43 °C q1h [Citation94]. They observed no significant changes in ATP or glucose, but levels of lactate increased 1.5-fold, supporting Lee et al.’s earlier work [Citation95]. Moon et al. examined the effects of 42 °C q1h heating on the metabolism of 4T1 cells [Citation76]. As indicated above, the group found that hyperthermia increased HIF-1 levels. Since HIF-1 is known to drive the switch to anaerobic metabolism via its regulation of most glycolytic enzymes [Citation68], this result is consistent with the observations of Streffer’s group. In fact, Moon et al. observed increased lactate levels in the serum of tumour-bearing mice after local heat treatment of tumours growing in the flank [Citation76]. The switch to anaerobic metabolism occurred in response to up-regulation of PDK-1 (pyruvate dehydrogenase lipoamide kinase isozyme 1), a master regulator of the balance between aerobic and anaerobic metabolism. Adding to the switch, however, was a substantial decrease in oxygen consumption rate, which occurred in response to a loss of mitochondrial membrane potential ().
Figure 6. Hyperthermia increases oxygenation via modulation of HIF-1 transcriptional activity. Moon et al. [Citation76] demonstrated that hyperthermia activates the ERK pathway, which leads to up-regulation of NADPH oxidase. NADPH activity increases intracellular superoxide anions, which then stimulates HIF-1 stabilisation. Reoxygenation follows as surviving tumour cells reduce oxygen consumption rate as they switch to aerobic glycolysis. In concert, hyperthermia decreases the mitochondrial membrane potential to further lower oxygen consumption and improve overall oxygenation levels.
![Figure 6. Hyperthermia increases oxygenation via modulation of HIF-1 transcriptional activity. Moon et al. [Citation76] demonstrated that hyperthermia activates the ERK pathway, which leads to up-regulation of NADPH oxidase. NADPH activity increases intracellular superoxide anions, which then stimulates HIF-1 stabilisation. Reoxygenation follows as surviving tumour cells reduce oxygen consumption rate as they switch to aerobic glycolysis. In concert, hyperthermia decreases the mitochondrial membrane potential to further lower oxygen consumption and improve overall oxygenation levels.](/cms/asset/4535f0b4-3b3f-46f0-8713-6c335b580c69/ihyt_a_1091093_f0006_c.jpg)
Future directions: Given the rapid increase in interest in tumour cell metabolism, this field is ripe for expansion for greater examination of hyperthermic effects and how they could be exploited in combination with a range of drugs that are targeting tumour metabolism.
Thermal ablation
We were not able to find any references where tumour metabolism has been studied after thermal ablation.
Total body hyperthermia
We were unable to identify any references using the MeSH terms of ‘total body hyperthermia’ or ‘fever-range hyperthermia’ and tumour metabolism. However, using the MeSH terms of ‘fever’ and ‘glycolysis’ we found several papers. These dealt with bacterial infections. The key link between hyperthermia and bacterial infections, such as those caused by salmonella, is their requirement of anaerobic metabolism for replication in macrophages, their natural host [Citation96].
Future directions: A potential emerging line of investigation could center on how fever plays into this pathogenesis.
Conclusion
It is clear that there is large potential for hyperthermia to play a central role in modifying tumour biology for potential therapeutic gain. The subjects discussed here represent the future of the field, but are not the only areas researchers would be wise to pursue. One of the fastest growing fields that could be applied is nanotechnology. For example, there are many papers showing that nanotechnology can enhance efficacy of vaccines and immune responsiveness to tumours [Citation97–99]. One paper has suggested that the combination of a nanoparticle-based gene therapy for Hsp70 with hyperthermia can further augment anti-tumour immunity [Citation100]. There are over 5900 papers identified by cross referencing the MeSH terms ‘hyperthermia’ with ‘cancer’. Surprisingly, the top cited papers on this list are related to nanotechology [Citation101]. Seven of the top 10 cited papers deal with magnetic or gold nanoparticles [Citation101–107]. Six of those seven have been categorised by Thomson Reuters as being ‘highly cited’, which means that they are in the top 1% of all cited papers in their categories. The 29th most highly cited paper, published in the International Journal of Hyperthermia, was by Jordan et al., on the subject of ferromagnetic fluids! [Citation108]. The International Journal of Hyperthermia has published two successful special issues on nanotechnology [Citation109,Citation110], but only a few of the scientists who study nanotechnology attend hyperthermia society meetings. Further, they do not publish routinely in this journal. Yet, knowledge of hyperthermia biology is essential for the ultimate applications and potential clinical success of nanotechnology. Efforts will be redoubled to increase interest in our societies and in our journal as a venue for publication of this highly meritorious field which holds great potential for clinical translation. We also anticipate that the role of inflammation in tumour hyperthermia treatments will be further characterised, as there has recently been great progress in this area using non-tumour models [Citation61,Citation111,Citation112].
Acknowledgements
The authors acknowledge the interactions with literally hundreds of scientists over the last 30+ years that have shaped their thinking about how hyperthermia can be used to improve cancer therapeutics. We thank all of them.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–74
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57–70
- Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 2015;742:53. PMID2605191
- Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin Oncol 2014;41:714–29
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, et al. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002;3:487–97
- Landon CD, Park J, Needham D, Dewhirst MW. Nanoscale drug delivery and hyperthermia: The materials design and preclinical and clinical testing of low temperature-sensitive liposomes used in combination with mild hyperthermia in the treatment of local cancer. Open Nanomed J 2011;3:38–64
- Koning GA, Eggermont AMM, Lindner LH, ten Hagen TLM. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res 2010;27:1750–4
- McDaniel JR, Dewhirst MW, Chilkoti A. Actively targeting solid tumours with thermoresponsive drug delivery systems that respond to mild hyperthermia. Int J Hyperthermia 2013;29:501–10
- Meyer DE, Shin BC, Kong GA, Dewhirst MW, Chilkoti A. Drug targeting using thermally responsive polymers and local hyperthermia. J Control Release 2001;74:213–24
- Lindner LH, Hossann M. Factors affecting drug release from liposomes. Curr Opin Drug Discov Devel 2010;13:111–23
- Nath K, Nelson DS, Heitjan DF, Leeper DB, Zhou R, Glickson JD. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed 2015;28:281–90
- Lee AH, Tannock IF. Heterogeneity of intracellular pH and of mechanisms that regulate intracellular pH in populations of cultured cells. Cancer Res 1998;58:1901–8
- Leeper DB, Engin K, Thistlethwaite AJ, Hitchon HD, Dover JD, Li DJ, et al. Human tumor extracellular ph as a function of blood-glucose concentration. Int J Radiat Oncol Biol Phys 1994;28:935–43
- Coss RA, Storck CW, Wells TC, Kulp KA, Wahl M, Leeper DB. Thermal sensitisation by lonidamine of human melanoma cells grown at low extracellular pH. Int J Hyperthermia 2014;30:75–8
- Nath K, Nelson DS, Heitjan DF, Zhou R, Leeper DB, Glickson JD. Effects of hyperglycemia on lonidamine-induced acidification and de-energization of human melanoma xenografts and sensitization to melphalan. NMR Biomed 2015;28:395–403
- Ahmed M, Monsky WE, Girnun G, Lukyanov A, D’Ippolito G, Kruskal JB, et al. Radiofrequency thermal ablation sharply increases intratumoral liposomal doxorubicin accumulation and tumor coagulation. Cancer Res 2003;63:6327–33
- Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia 2004;20:781–802
- Goldberg SN, Kamel IR, Kruskal JB, Reynolds K, Monsky WL, Stuart KE, et al. Radiofrequency ablation of hepatic tumors: Increased tumor destruction with adjuvant liposomal doxorubicin therapy. Am J Roentgenol 2002;179:93–101
- Wong C, Stylianopoulos T, Cui JA, Martin J, Chauhan VP, Jiang W, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci USA 2011;108:2426–31
- Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res 2011;71:3872–80
- Xu Y, Choi J, Hylander B, Sen A, Evans SS, Kraybill WG, et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int J Hyperthermia 2007;23:513–27
- Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst 2015;107:djv040
- Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Applied Physiol 2012;113:263–72
- Dayanc BE, Bansal S, Gure AO, Gollnick SO, Repasky EA. Enhanced sensitivity of colon tumour cells to natural killer cell cytotoxicity after mild thermal stress is regulated through HSF1-mediated expression of MICA. Int J Hyperthermia 2013;29:480–90
- Barnes KD, Shafirstein G, Webber JS, Koonce NA, Harris Z, Griffin RJ. Hyperthermia-enhanced indocyanine green delivery for laser-induced thermal ablation of carcinomas. Int J Hyperthermia 2013;29:474–9
- Mikucki ME, Fisher DT, Ku AW, Appenheimer MM, Muhitch JB, Evans SS. Preconditioning thermal therapy: Flipping the switch on IL-6 for anti-tumour immunity. Int J Hyperthermia 2013;29:464–73
- Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza APD, Bonorino C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBP beta and C/EBP delta. Int J Hyperthermia 2013;29:455–63
- Van Herwijnen MJC, Van der Zee R, Van Eden W, Broere F. Heat shock proteins can be targets of regulatory T cells for therapeutic intervention in rheumatoid arthritis. Int J Hyperthermia 2013;29:448–54
- Calderwood SK. From stress protein biochemistry to novel immunotherapeutics. Int J Hyperthermia 2013;29:362–3
- Calderwood SK, Gong J, Stevenson MA, Murshid A. Cellular and molecular chaperone fusion vaccines: Targeting resistant cancer cell populations. Int J Hyperthermia 2013;29:376–9
- Murshid A, Eguchi T, Calderwood SK. Stress proteins in aging and life span. Int J Hyperthermia 2013;29:442–7
- Torigoe T, Hirohashi Y, Yasuda K, Sato N. Constitutive expression and activation of stress response genes in cancer stem-like cells/tumour initiating cells: Potent targets for cancer stem cell therapy. Int J Hyperthermia 2013;29:436–41
- Csoboz B, Balogh GE, Kusz E, Gombos I, Peter M, Crul T, et al. Membrane fluidity matters: Hyperthermia from the aspects of lipids and membranes. Int J Hyperthermia 2013;29:491–9
- Singh IS, Hasday JD. Fever, hyperthermia and the heat shock response. Int J Hyperthermia 2013;29:423–35
- Arrigo A-P, Gibert B. Protein interactomes of three stress inducible small heat shock proteins: HspB1, HspB5 and HspB8. Int J Hyperthermia 2013;29:409–22
- Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int JHyperthermia 2013;29:399–408
- Epple LM, Bemis LT, Cavanaugh RP, Skope A, Mayer-Sonnenfeld T, Frank C, et al. Prolonged remission of advanced bronchoalveolar adenocarcinoma in a dog treated with autologous, tumour-derived chaperone-rich cell lysate (CRCL) vaccine. Int J Hyperthermia 2013;29:390–8
- Graner MW, Romanoski A, Katsanis E. The ‘peptidome' of tumour-derived chaperone-rich cell lysate anti-cancer vaccines reveals potential tumour antigens that stimulate tumour immunity. Int JHyperthermia 2013;29:380–9
- Mayer-Sonnenfeld T, Har-Noy M, Lillehei KO, Graner MW. Proteomic analyses of different human tumour-derived chaperone-rich cell lysate (CRCL) anti-cancer vaccines reveal antigen content and strong similarities amongst the vaccines along with a basis for CRCL's unique structure: CRCL vaccine proteome leads to unique structure. Int J Hyperthermia 2013;29:520–7
- Wang X-Y, Subjeck JR. High molecular weight stress proteins: Identification, cloning and utilisation in cancer immunotherapy. Int J Hyperthermia 2013;29:364–75
- Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res 2013;1:210–16
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9
- Viglianti BL, Dewhirst MW, Boruta RJ, Park JY, Landon C, Fontanella AN, et al. Systemic anti-tumour effects of local thermally sensitive liposome therapy. Int J Hyperthermia 2014;30:385–92
- Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med 2007;5:34
- Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res 2012;72:430–9
- Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 2006;66:1139–46
- Takagi H, Azuma K, Osaki T, Itoh N, Nakazumi S, Taura Y, et al. High temperature hyperthermia treatment for canines exhibiting superficial tumors: A report of three cases. Oncol Lett 2014;8:2055–8
- Liu Q, Zhai B, Yang W, Yu LX, Dong W, He YQ, et al. Abrogation of local cancer recurrence after radiofrequency ablation by dendritic cell-based hyperthermic tumor vaccine. Mol Ther 2009;17:2049–57
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res 2005;127:208–23
- Kroeze SGC, van Melick HHE, Nijkamp MW, Kruse FK, Kruijssen LWJ, van Diest PJ, et al. Incomplete thermal ablation stimulates proliferation of residual renal carcinoma cells in a translational murine model. BJU Int 2012;110:E281–6
- Manzoor AA, Lindner LH, Landon CD, Park J-Y, Simnick AJ, Dreher MR, et al. Overcoming Limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res 2012;72:5566–75
- Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest 2011;121:3846–59
- Appenheimer MM, Chen Q, Girard RA, Wang WC, Evans SS. Impact of fever-range thermal stress on lymphocyte-endothelial adhesion and lymphocyte trafficking. Immunol Investig 2005;34:295–323
- Haveman J, Geerdink AG, Rodermond HM. Cytokine production after whole body and localized hyperthermia. Int J Hyperthermia 1996;12:791–800
- Wang XY, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol 2001;166:490–7
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252–64
- Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263–6
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Investig 2014;124:5466–80
- Ostberg JR, Ertel BR, Lanphere JA. An important role for granulocytes in the thermal regulation of colon tumor growth. Immunol Invest 2005;34:259–72
- Capitano ML, Ertel BR, Repasky EA, Ostberg JR. Winner of the 2007 Society for Thermal Medicine Young Investigator Award. Fever-range whole body hyperthermia prevents the onset of type 1 diabetes in non-obese diabetic mice. Int J Hyperthermia 2008;24:141–9
- Lee CT, Kokolus KM, Leigh ND, Capitano M, Hylander BL, Repasky EA. Defining immunological impact and therapeutic benefit of mild heating in a murine model of arthritis. PLoS One 2015;10:e0120327
- Aird KM, Allensworth JL, Batinic-Haberle I, Lyerly HK, Dewhirst MW, Devi GR. ErbB1/2 tyrosine kinase inhibitor mediates oxidative stress-induced apoptosis in inflammatory breast cancer cells. Breast Cancer Res Treat 2012;132:109–19
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009;30:1073–81
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem-Biol Interact 2006;160:1–40
- Ali F, Sultana S. Repeated short-term stress synergizes the ROS signalling through up regulation of NFkB and iNOS expression induced due to combined exposure of trichloroethylene and UVB rays. Mol Cell Biochem 2012;360:133–45
- Gorlach A, Berchner-Pfannschmidt U, Wotzlaw C, Cool RH, Fandrey J, Acker H, et al. Reactive oxygen species modulate HIF-1 mediated PAI-1 expression: Involvement of the GTPase Rac1. Thromb Haemost 2003;89:926–35
- Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, et al. Regulation of HIF-1 alpha stability through S-nitrosylation. Mol Cell 2007;26:63–74
- Semenza GL. Mechanisms of disease oxygen sensing, homeostasis, and disease. N Engl J Med 2011;365:537–47
- Dewhirst MW. Relationships between cycling hypoxia, HIF-1, angiogenesis and oxidative stress. Radiat Res 2009;172:653–65
- Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 2008;8:425–37
- Ye XD, Fels D, Tovmasyan A, Aird KM, Dedeugd C, Allensworth JL, et al. Cytotoxic effects of Mn(III) N-alkylpyridylporphyrins in the presence of cellular reductant, ascorbate. Free Radic Res 2011;45:1289–306
- Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia 2014;30:513–23
- Davidson JF, Whyte B, Bissinger PH, Schiestl RH. Oxidative stress is involved in heat-induced cell death in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 1996;93:5116–21
- Mitchell JB, Russo A. Thiols, thiol depletion, and thermosensitivity. Radiat Res 1983;95:471–85
- Laskowitz DT, Elion GB, Dewhirst MW, Griffith OW, Casero RA, Scott PA, et al. effects of glutathione or polyamine depletion on in vivo thermosensitization. Int J Hyperthermia 1992;8:199–208
- Moon EJ, Sonveaux P, Porporato PE, Danhier P, Gallez B, Batinic-Haberle I, et al. NADPH oxidase-mediated reactive oxygen species production activates hypoxia-inducible factor-1 (HIF-1) via the ERK pathway after hyperthermia treatment. Proc Natl Acad Sci USA 2010;107:20477–82
- Nie W, Ma XL, Sang YX, Li YL, Gao X, Xu GC, et al. Synergic antitumor effect of SKLB1002 and local hyperthermia in 4T1 and CT26. Clin Exper Med 2014;14:203–13
- Chen P, Yang LL, Yang HS, Wang YS, Li G, Wu Y, et al. Synergistic antitumor effect of CXCL10 with hyperthermia. J Cancer Res Clin Oncol 2008;134:679–87
- Roca C, Primo L, Vaidembri D, Cividalli A, Declerck P, Carmeliet P, et al. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor 1-dependent mechanism. Cancer Res 2003;63:1500–7
- Moon EJ, Brizel DM, Chi JTA, Dewhirst MW. The potential role of intrinsic hypoxia markers as prognostic variables in cancer. Antioxid Redox Signal 2007;9:1237–94
- Cao W, Xu X, Zhang JL, Duan YY. Tumor angiogenesis after heated lipiodol infusion via the hepatic artery in a rabbit model of VX2 liver cancer. PLoS One 2013;8:e61583
- Kong J, Kong JG, Pan B, Ke S, Dong SY, Li XL, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1 alpha/VEGFA. PLoS One 2012;7(5)
- Nikfarjam M, Muralidharan V, Christophi C. Altered growth patterns of colorectal liver metastases after thermal ablation. Surgery. 2006;139:73–81
- Gong B, Asimakis GK, Chen ZP, Albrecht TB, Boor PJ, Pappas TC, et al. Whole-body hyperthermia induces up-regulation of vascular endothelial growth factor accompanied by neovascularization in cardiac tissue. Life Sci 2006;79:1781–8
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA. 2011;108:9851–6
- Pelicci PG, Dalton P, Orecchia R. Heating cancer stem cells to reduce tumor relapse. Breast Cancer Res 2011;13:305
- Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, et al. Thermal Enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med 2010;2:55ra79
- Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, et al. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One 2014;9(2):e87979
- Yang Y, Qu B, Huo JH, Wu SL, Zhang MY, Wang ZR. Serum from radiofrequency-injured livers induces differentiation of bone marrow stem cells into hepatocyte-like cells. J Surg Res 2009;155:18–24
- Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA 2010;107:8363–8
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010;120:694–705
- Capitano ML, Nemeth MJ, Mace TA, Salisbury-Ruf C, Segal BH, McCarthy PL, et al. Elevating body temperature enhances hematopoiesis and neutrophil recovery after total body irradiation in an IL-1-, IL-17-, and G-CSF-dependent manner. Blood 2012;120:2600–9
- Tamulevicius P, Gobel D, Streffer C. Effect of hyperthermia on energy-metabolism in vivo. Strahlentherapie 1983;159:383–4
- Tamulevicius P, Streffer C. Bioluminescence imaging of metabolites in a human tumour xenograft after treatment with hyperthermia and/or the radiosensitizer pimonidazole. Int J Hyperthermia 1997;13:235–45
- Lee SY, Ryu KH, Kang MS, Song CW. Effect of hyperthermia on the lactic acid and beta-hydroxybutyric acid content in tumour. Int J Hyperthermia 1986;2:213–22
- Bowden SD, Rowley G, Hinton JCD, Thompson A. Glucose and glycolysis are required for the successful infection of macrophages and mice by Salmonella enterica serovar Typhimurium. Infect Immun 2009;77:3117–26
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J Immunol 2004;173:3148–54
- Hadrup SR, Bakker AH, Shu CJ, Andersen RS, van Veluw J, Hombrink P, et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat Methods 2009;6:520–6
- Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Del Rev 2008;60(8):915–28
- Ito A, Matsuoka F, Honda H, Kobayashi T. Heat shock protein 70 gene therapy combined with hyperthermia using magnetic nanoparticles. Cancer Gene Ther 2003;10:918–25
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005;26:3995–4021
- Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J] Biosci Bioeng 2005;100:1–11
- Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mat 1999;201:413–19
- Mornet S, Vasseur S, Grasset F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J Mater Chem 2004;14:2161–75
- Neuberger T, Schopf B, Hofmann H, Hofmann M, von Rechenberg B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn Magn Mater 2005;293:483–96
- Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2003;36:R167–81
- Skrabalak SE, Chen JY, Sun YG, Lu XM, Au L, Cobley CM, et al. Gold nanocages: Synthesis, properties, and applications. Accounts Chem Res 2008;41:1587–95
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids – physical evaluation of their potential for hyperthermia. Int J Hyperthermia 1993;9:51–68
- Dewhirst MW. Hyperthermia and nanotechnology – a note from the Editor-in-chief. Int J Hyperthermia 2008;24:449–50
- Ivkov R. Magnetic nanoparticle hyperthermia: A new frontier in biology and medicine? Int J Hyperthermia 2013;29:703–5
- Tarner IH, Muller-Ladner U, Uhlemann C, Lange U. The effect of mild whole-body hyperthermia on systemic levels of TNF-alpha, IL-1beta, and IL-6 in patients with ankylosing spondylitis. Clin Rheumatol 2009;28:397–402
- Tulapurkar ME, Hasday JD, Singh IS. Prolonged exposure to hyperthermic stress augments neutrophil recruitment to lung during the post-exposure recovery period. Int J Hyperthermia 2011;27:717–25
