Abstract
Purpose: Radiofrequency-based electrophysical agents are widely used in therapy-related clinical practice for their thermal effects, mainly relieving pain and inflammation and improving tissue extensibility. The most commonly used and researched are shortwave therapies that operate at 27.12 MHz. Although relatively new, electrophysical agents employing much lower frequencies have also emerged. Capacitive resistive monopolar radiofrequency employing 448 kHz is one such therapy. This laboratory-based study was aimed to investigate the skin thermal responses to 448 kHz radiofrequency-based therapy in healthy adults.Methods: In a two-group randomised crossover study, 15 volunteers attended two modes (capacitive and resistive) of 448 kHz radiofrequency-based therapy (using ‘Indiba Activ 902’) administered locally to the lower thigh region. Starting at minimum, the intensity was increased incrementally until thermal discomfort was felt. Participants reported three time points: thermal onset, definite thermal sensation, and onset of thermal discomfort. Local skin temperature was measured before, immediately post-treatment and up to 45 min post-treatment.Results: Both capacitive and resistive modes of therapy significantly increased the skin temperature and sustained it over the 45-min follow-up. There was statistically significant difference between the thermal response patterns produced by the two modes. Peak post-treatment temperatures attained were not significantly different between the two; however, the retention rate at follow-up was significantly higher for the resistive mode.Conclusions: This study confirms that radiofrequency-based therapy at 448 kHz can significantly increase and sustain skin temperature. The study also provides useful baseline data for further research in the low frequency ranges of radiofrequency-based therapy that remain largely unexplored.
Introduction
Electrophysical agents (EPAs) are used by therapists to treat a wide variety of conditions. Some of these agents can induce hyperthermia in tissues without being invasive or ablative. While some produce superficial heating to the area of the body where the modality is applied (for example, infrared (IR) therapy), others such as radiofrequency electromagnetic field (RFEMF, or simply RF)-based EPAs are capable of heating the skin as well as deeper structures (such as muscles and joint tissues) [Citation1].
The physiological effects of heat on living tissues are well documented. Heat can induce changes in the superficial and deep tissues, at cellular and systemic levels. A modest rise in temperature (mild hyperthermia) is sufficient to accelerate and/or increase most cellular activity, and heat-induced vasodilatation can enhance local blood circulation in the tissues [Citation1–3]. Heat can also change the nature of connective tissues. It can alter the properties of tendons, ligaments and, to some extent muscle, by means of increasing the extensibility and reducing the tone and spasm [Citation1–3]. The extent of physiological effect may vary depending on the level of exposure.
In therapy, heat application is often used as a mode to relieve pain and inflammation and potentially enhance tissue healing. Various physiological mechanisms are believed to underpin the effects of heat on pain and tissue repair including but not limited to changes arising from an increase in the blood flow, oxygen uptake and chemical reaction rates. Therapeutically, a rise in tissue temperature by more than 1 °C will help to relieve mild inflammation and an increase of 2–3 °C will help to reduce pain and muscle spasm, whereas an increase of 3–4 °C can produce changes in tissue extensibility [Citation3,Citation4]. A temperature rise of this range employed in therapy-related clinical practice is only ‘mild hyperthermia’ whilst in many other invasive and cytotoxic procedures in medicine (e.g. radiofrequency ablation) the rise is substantially higher.
Radiofrequency-based EPAs have been employed for various levels of heat treatment in therapy practice since the early decades of the last century [Citation5]. The electromagnetic energy delivered by these devices generates heat in the tissues as a result of the oscillation and friction of charged molecules (such as proteins and ions). The rise in temperature itself is dependent upon the electrical properties of the tissues and the rate at which the energy is absorbed (specific absorption rate, SAR) [Citation1,Citation6].
Among the EPAs that are used to induce mild hyperthermia, longwave diathermy (which employed RF fields of around 0.5–1 MHz) became obsolete in the 1950s due to practical limitations and the severe disturbances it caused to communication and broadcasting [Citation7]. Shortwave therapy (SWT) (also known as shortwave diathermy, or SWD), which commonly operates at 27.12 MHz, and microwave therapy (also known as microwave diathermy, or MWD), which operates at up to 2.45 GHz became more established in practice. However, microwave therapy is currently used infrequently in many countries [Citation8,Citation9].
Shortwave therapy is used in two modes: continuous (CSWT) and pulsed (PSWT). CSWT has been widely used by clinicians since the 1930s but has now become less popular and is infrequently used in the western world [Citation8,Citation10]. Similar to microwave therapy, CSWT typically causes relatively higher levels of heating in the tissues compared to PSWT as it does not allow an adequate ‘thermal washout’ during the treatment [Citation1]. The diminishing popularity of CSWT is linked partly to a ‘fashionable shift’ rather than solely to published evidence because an evidence base does exist in support of the use of CSWT [Citation11]. By contrast, PSWT, which was developed in the 1940s, has since become the more popular delivery option and is still used widely [Citation8,Citation10,Citation12].
The majority of research on the biological effects of RF is centred on the higher frequency (microwaves), particularly areas such as mobile telephony [Citation13]. However, a recent review of literature undertaken by the authors of this study [Citation14] indicated that the RF currently used in therapy-related clinical practice is predominantly within the relatively lower frequency range of 30 kHz–30 MHz and largely limited to the shortwave range of 10–30 MHz (especially PSWT at 27.12 MHz as a delivery mode) [Citation8,Citation10,Citation11].
Although the research is sparse, EPAs operating at significantly lower RF ranges (< 1 MHz) have also been reported and used in clinical practice to induce mild hyperthermia and other physiological effects [Citation15–18]. An example for such EPAs currently used in therapy practice is capacitive resistive monopolar radiofrequency (CRMRF), which operates at a frequency of 448 kHz. In this study the authors aimed to investigate the skin thermal responses (thermal effects) to the cutaneous application of continuous mode CRMRF therapy in healthy adults.
Materials and methods
Apparatus
The CRMRF energy was delivered using an ‘Indiba Activ 902’ device (Indiba S. A., Barcelona, Spain). The equipment was factory calibrated and pretested for accuracy of output. The peak power of the device was 200 W (or 450 VA (Volt-Amp)). It delivers RF energy in two modes: capacitive (CAP) and resistive (RES), the intensities of which are given as percentage output.
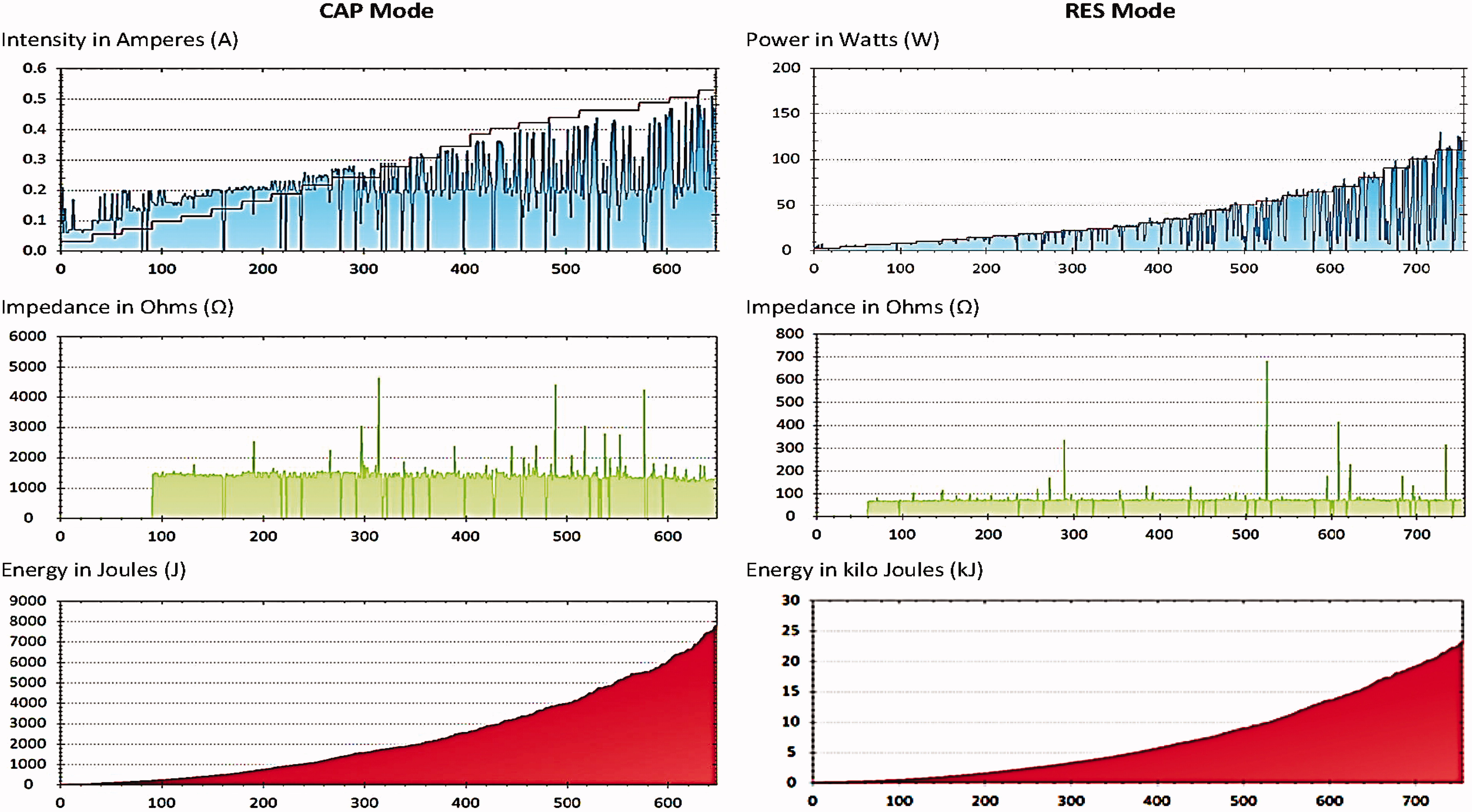
Rigid circular metallic electrodes with a diameter of 65 mm were used as active electrodes and a large flexible rectangular metallic plate (measuring 200 × 260 mm) was used as the inactive electrode. A manufacturer-supplied conductive cream was employed as a coupling medium between the electrode and the skin surface. The CAP electrode has a polyamide coating that acts as a dielectric medium, insulating its metallic body from the skin surface, thus forming a capacitor with the treated tissues. The RES electrode is uncoated and passes RF energy directly through the body and into the neutral plate. The RF intensity and the energy output () is shown on the machine display, which is also wirelessly recorded by a computer-based monitoring software programme in the research environment. A detailed log of the experimental data can be exported via the monitoring software.
Figure 1. Graphs showing the output data as obtained from the device monitoring software. The data shown are from one participant (No. 8). The x-axis shows the monitoring sequence units (more than 1/s) and y-axis shows the parameter recorded.
A hand-held contactless IR skin surface thermometer Tecnimed Srl, Vedano Olona, Italy (Thermofocus 01500A3) and an IR tympanic thermometer Braun GmbH, Kronberg, Germany (Braun ThermoScan IRT 4520) were used for skin surface and core temperature measurements respectively. The surface thermometer has a measurement accuracy of ± 0.3 °C between 20.0–42.5 °C, with an improved accuracy of ± 0.2 °C between 36.0–39.0 °C. The tympanic thermometer has a measurement accuracy of ± 0.2 °C between 35.5–42.0 °C, and ± 0.3 °C outside of this range. A body composition monitor Omron Healthcare Europe B.V., Hoofddorp, Netherlands (Omron BF508) was used to obtain the weight, body fat percentage, visceral fat and the body mass index (BMI) measurements of the participants. An electronic metronome (Seiko Instruments Inc., Tokyo, Japan) and a computer-based timer were used to provide 1-s and 30-s beeps respectively in order to aid the experimental procedure. The room temperature and humidity were monitored using an electronic thermohygrometer RS Components Pte Ltd., Singapore (RS 212–124).
Sample and groups
A randomly selected cohort of 15 asymptomatic (self-reported) adults from among the 27 000 staff and students of the University of Hertfordshire participated in the study. The recruitment was done through e-mails that were sent out university-wide. The respondents were screened for inclusion consecutively, and the first 15 eligible subjects were recruited (eligibility criteria explained below). All participants signed an informed consent prior to the study. The study was approved by the Health and Human Sciences Ethics Committee with Delegated Authority (HHSECDA) of the University of Hertfordshire (Protocol number: HSK/PG/UH/00015).
Each participant attended two sessions; one each for the CAP and the RES modes thus forming two separate treatment groups. The order of attendance was randomised by concealment using a computer-generated randomisation chart (IBM SPSS Statistics, Version 20) and blinded from the participant. There were no control or placebo groups. Based on pilot experiments, a minimum gap of 48 h was allowed between the two sessions so that no residual effects from the first session were present at the time of the second. Similar times of the day (± 1 h) were chosen for either session for each participant.
Experimental procedure
On the day of the tests, the participants were asked to avoid food, beverages and strenuous exercises within 1 h before the start of the experiment to ensure that their physiological condition remained stable during the sessions. The participants were screened using an eligibility questionnaire including questions relating to any recent injury or illness and accepted contraindications to RF therapy (pregnancy, malignancy, metal or electronic implants in the body). Subsequently, their ‘skin thermal sensitivity’ was tested using test tubes filled with water at approximately three different temperatures (± 0.5 °C): 45 °C (warm), 35 °C (neutral) and 25 °C (cold). The participants were required to distinguish between the three temperatures in order to continue with their participation in the study. After this screening, demographic and anthropometric data were collected.
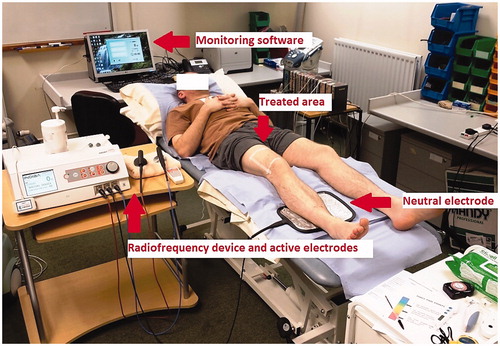
To receive treatment, the participants were asked to lie down in a supine position on a treatment plinth and were fully supported with pillows (). A square area covering the lower quarter of the anterior aspect of the right thigh was marked with tape. The local skin temperature was recorded from the centre of the marked square area on the treated leg and the corresponding area of the untreated leg. The untreated leg served as a control. The core (tympanic) temperature was also concurrently monitored. The local skin temperature measurements were repeated every 2 min until it stabilised, which was then considered the baseline skin temperature. The neutral plate electrode was smeared with 20 ml of conductive cream and placed under the calf muscle belly, one quarter of the way down the distance from the fibular head to the lateral malleolus of the treated leg.
Figure 2. The experimental setting with the 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) device (Indiba Activ 902), electrodes and the computer-based monitoring software.
The CRMRF treatment was applied with the active electrode using 20 ml of cream in the marked square area. The intensity started at the lowest level permitted by the device and was raised by one level every 30 s. The active electrode produced firm circles on the skin, at a rate of ‘one per second’ as guided by the metronome. The participants were instructed to report clearly and promptly at three time points: Firstly, when they start to feel heat on the skin (thermal onset), secondly, when they feel moderate yet comfortable heat (definite thermal sensation), and thirdly, when the heat starts to become uncomfortable (onset of thermal discomfort). All three time points and the corresponding treatment intensity were recorded. The treatment was stopped once the ‘onset of thermal discomfort’ was reached.
After clearing the treated area, the post-treatment (peak) skin temperature was recorded from either leg from the same spot used for the baseline measurement. The core temperature was also recorded at this time. The skin temperature measurements were repeated (follow-up) subsequently every 30 s on the treated leg and every 5 min on the control leg for the next 45 min, or till the temperature on the treated side returned to the baseline level (whichever was earlier). Core temperature, room temperature and humidity measurements were also repeated at the end of the experiment.
In addition, a second brief experiment was conducted separately, to map the temperature changes in the active treatment electrodes at various stages of testing in response to set intensities (expressed as percentages) of application of the CRMRF energy. For this purpose three arbitrary intensity levels were chosen, alongside a fourth level which would be the mean peak power reached during each mode of the main experiment.
Data analysis
All data was processed and analysed using Microsoft Office Excel (Version 2010, Microsoft Corporation) and IBM SPSS Statistics (Version 20) for Windows. The group data were compared using a two-way repeated measures analysis of variance (ANOVA) model with a Bonferroni post-hoc comparison for the thermal responses of the CAP and RES modes at three time points (baseline, post-treatment and 45-minute follow-up). The statistical significance was set at p ≤ 0.05 (0.8 P, 95% CI).
Results
All 15 participants completed both sessions of the study and the assessments as anticipated. The RF treatment was well tolerated and there were no reports of any adverse events that may be a consequence of the intervention, including any issues due to potential overheating. The demographic and the mean ( ± SD) anthropometric data from the participants are reported in .
Table 1. Demographic and mean (±SD) anthropometric data from the 15 participants who received localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment.
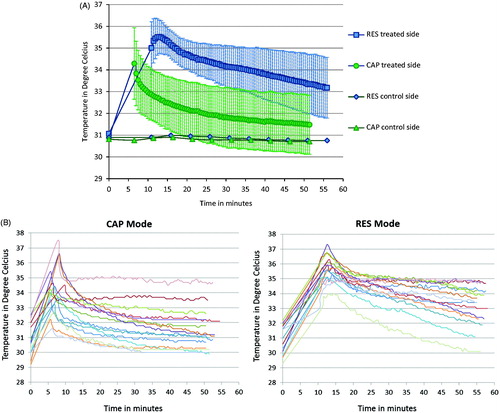
The mean (± SD) skin thermal responses as well as individual thermal response patterns obtained from all 15 participants to both CAP and RES modes of CRMRF therapy are plotted in ), ) and ). The baseline, post-treatment and 45-min follow-up mean (± SD) skin temperatures obtained for the CAP mode were 30.9 (± 1.1) °C, 34.3 (± 1.6) °C and 31.5 (± 1.4) °C respectively, and for the RES mode were 31.1 (± 1.0) °C, 35.0 (± 1.2) °C and 33.2 (± 1.4) °C respectively.
Figure 3. (A) Capacitive (CAP) and resistive (RES) mode mean skin thermal responses to localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment. The data shown (baseline, post-treatment and 45-min follow-up) are from 15 participants. *Statistically significant differences when compared to the baseline (at p < 0.05) (two-way repeated measures ANOVA). (B) Capacitive (CAP) and resistive (RES) mode individual skin thermal responses to localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side only). The data shown (baseline, post-treatment and 45-min follow-up) are from 15 participants.
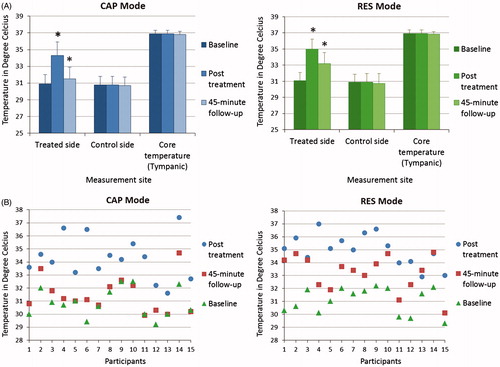
Figure 4. (A) Capacitive (CAP) and resistive (RES) mode mean skin thermal changes after localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side). The data shown (thermal build-up, thermal decay and thermal retention) are the changes in relation to the baseline from 15 participants. *Statistically significant differences when compared to the baseline (at p < 0.05) (two-way repeated measures ANOVA). (B) Capacitive (CAP) and resistive (RES) mode individual skin thermal changes after localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side). The data shown (thermal build-up, thermal decay and thermal retention) are the changes in relation to the baseline from 15 participants.
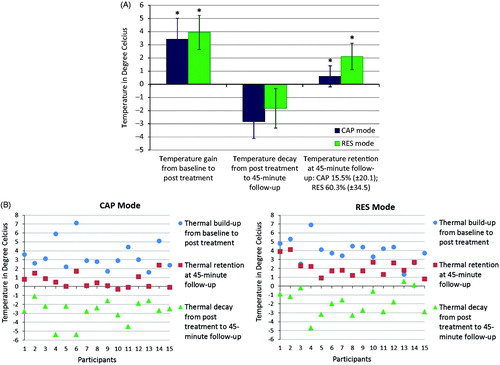
Figure 5. (A) Capacitive (CAP) and resistive (RES) mode mean skin thermal decay after localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side). The data are from 15 participants, showing the decay process from post treatment to 45-min follow-up. (B) Capacitive (CAP) and resistive (RES) mode individual skin thermal decay after localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side). The data are from 15 participants, showing the decay process from post treatment to 45-min follow-up.
shows the mean mean (± SD) time taken, energy delivered and the peak power output to reach each thermal stage. ) shows the individual data for the mean time, mean energy and mean peak power reported in .
Figure 6. (A) Capacitive (CAP) and resistive (RES) mode time-specific individual skin thermal responses to localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side only). The data shown (thermal onset, definite thermal and thermal discomfort) are from 15 participants. (B) Capacitive (CAP) and resistive (RES) mode energy-specific individual skin thermal responses to localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side only). The data shown (thermal onset, definite thermal and thermal discomfort) are from 15 participants. (C) Capacitive (CAP) and resistive (RES) mode power (peak)-specific individual skin thermal responses to localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment (treated side only). The data shown (thermal onset, definite thermal and thermal discomfort) are from 15 participants.
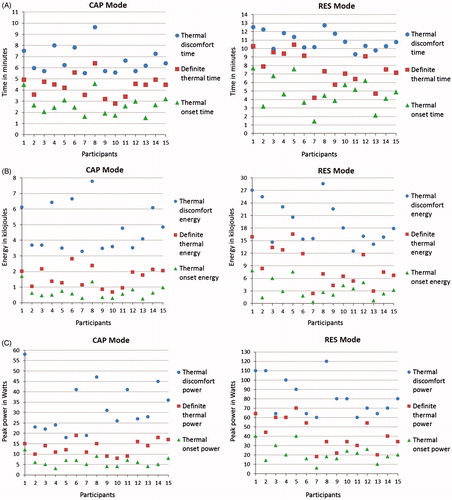
Table 2. Mean time, mean energy and mean peak power reached at each of the three thermal stages for 15 participants who received localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment.
There was no significant difference between the baseline skin temperatures of the two groups. Overall, there was statistically significant difference between the thermal response patterns produced by the CAP and RES modes (F (1, 14) = 16.639, p = 0.001) although their peak skin temperatures obtained at the end of treatment were not significantly different. From baseline to peak there was 11.1% increase in the mean skin temperature of CAP mode, and 12.7% increase in that of RES mode.
Both CAP and RES modes also showed a significant retention of the gained temperature at the 45-min follow-up compared to their baseline (CAP: F (1, 14) = 8.690, p = 0.011; RES: F (1, 14) = 70.321, p < 0.001). However, the rate of temperature retention at the 45-min follow-up was significantly higher for the RES mode compared to that of the CAP mode (53.6% and 17.5% retention respectively) (F (1, 14) = 36.173, p < 0.001).
A post-hoc power analysis using G*Power (Version 3.1) confirmed the actual power of the obtained ANOVA results to be 1.0. No meaningful change was noted in the thermal response of either the untreated control side or the core (tympanic) temperature at any time point in either group.
The temperature changes recorded from the two active electrodes during the second experiment are reported in .
Table 3. Electrode (active) temperature at various times and intensity of application during localised 448 kHz capacitive resistive monopolar radiofrequency (CRMRF) treatment. The study was conducted on a single male participant, aged 37 years (body mass index, 23) at room temperature 25 °C and humidity 66%.
Discussion
The literature surrounding the thermophysiological effects of RF on biological systems is extensive. Most of the published research in this area has been conducted on laboratory animals, the emphasis being particularly on rodents. It has been argued that these small mammals are poor models for human beings as their physiological heat loss mechanisms are limited, thus making extrapolation of the results from such studies to human beings difficult [Citation6]. For example, rodents (mice in particular) have a higher thermoneutral zone of around 30 °C whereas for humans (wearing clothes) it is 22–25 °C [Citation19–21]. In the present experiment, the mean (± SD) room temperature was 24.0 (± 0.8) °C for the CAP mode and 23.9 (± 1.2) °C for the RES mode, both of which are within the thermoneutral zone of humans.
Whether or not RF exposure to the human body produces a thermal effect depends on many parameters of the wave; such as its frequency, intensity, duration of exposure and the area of the body exposed. In a clinical environment, a prediction of this thermal response based on the existing models alone is challenging. Experimental and theoretical work suggests that at low intensity levels, ‘demodulation’ of RF is only practical at frequencies that are much lower than a few MHz. In other words, the biological systems cannot ‘rectify’ RF fields above a few MHz efficiently enough to affect the endogenous fields and the biological processes [Citation13].
Radiofrequency waves may interact with the biological tissues through a number of mechanisms. While the theory underpinning the thermal effects of RF interaction is well established, arguably the claims about the ‘so called’ low level non-thermal (LLNT) effects are somewhat controversial and lack sufficient experimental support [Citation13]. Although the non-thermal interaction mechanisms may not be directly associated with a temperature rise in the tissues, thermal effects do occur even at very low levels because all interactions between the RF fields and the biological tissues will result in energy transfer to the tissues, ultimately leading to an increase in temperature [Citation22]. For example, cell metabolism can be affected by changes in temperature that are as small as a fraction of a degree [Citation13]. However, as identified, many of the physiological benefits of heating occur when temperatures are raised by at least 2 °C.
The extent of physiological effects induced by the heating modalities (EPAs) depends on the type of energy delivered by the EPA and its depth of penetration [Citation23]. For example, it is believed that exposure to pulsed RF is more likely to induce certain biological effects than continuous RF at the same average incident pulse density. Similarly, while the low penetrative IR energy (non-RF) is absorbed in the most superficial layers of skin leading to superficial heating, RF frequencies will be absorbed in complex patterns at other depths [Citation6], potentially causing deeper heating and hence deeper effects. Although not measured in this study, other researchers have reported that deep heating may be attained using RF-based EPAs, and is more effective in inducing certain physiological effects such as increasing tissue extensibility, compared to superficial heating [Citation24,Citation25].
The results of the present study show significant differences between the patterns of heating produced by the CAP and RES modes of CRMRF therapy, as measured using a skin thermometer. The CAP mode response in relation to the three thermal stages (onset, definite and discomfort) was achieved considerably faster, but the built-up temperature decayed faster and was retained less compared to that of the RES mode. However, the lack of linearity between the intensity settings of the two modes renders any further comparison problematic. Similarly, the peak power achieved at each time point would also have been influenced by the lack of linearity. The individual data () suggests that for either mode the greater the temperature increase due to heating, the greater was the drop during the follow-up phase.
As stated, the mean (± SD) peak skin temperature obtained was 34.3 (± 1.6) °C for the CAP mode and 35.0 (± 1.2) °C for the RES mode (). The highest temperature recorded for any particular participant was 37.4 °C for the CAP mode and 37.0 °C for the RES mode (). Per se, these values are below or barely at the level of the core temperature. Hence, what is achieved is only ‘mild hyperthermia’ at best, and solely in relation to the baseline skin temperatures recorded at the treatment site. To be termed hyperthermia, the temperature would have to be raised to supra-physiological levels, that is, to around 40–45 °C, or even higher for thermal ablation. As in vitro cell studies have shown, the cytotoxic effects of heat and cell death are prominent over a temperature range of 40–55 °C, with a ‘break point’ at around 43 °C [Citation26–29].
A cumulative or equivalent thermal dose is often used to quantify the thermal doses given during heat treatments [Citation30]. The cumulative or total equivalent thermal dose can be expressed as cumulative equivalent minutes (CEM) in relation to the arbitrarily chosen temperature of 43 °C (CEM 43), as originally proposed by Sapareto and Dewey [Citation31]. Using this method any ‘time–temperature’ history can be converted to a single number that represents a ‘thermal isoeffect dose’, which is an equivalent number of minutes of heating at 43 °C. The following formula is used for this calculation if the temperature remained constant:
where CEM 43 °C is the cumulative number of equivalent minutes at 43°C, t is the time (duration) of treatment, R is a constant related to the temperature dependence of the rate of cell death (R (T < 43 °C) = 0.25, R (T > 43 °C) = 0.5) and T is the temperature during t.
For a complex time–temperature history, the CEM 43 °C is calculated for each small time interval (t) where the temperature (T) has remained relatively constant and summated over the entire period using the formula:
where ‘Tavg’ is the average temperature for each time interval (t). The resulting CEM 43 °C value represents the effect of the entire history of heat exposure.
Estimates of ‘thermal isoeffect dose’ are predominantly used in the dosimetry of cancer thermotherapy and of magnetic resonance (MR) systems [Citation30]. It can also be performed for CRMRF therapy, since studies employing CRMRF to induce hyperthermia for cancer [Citation18,Citation32–34] and other cellular effects [Citation17] have been reported.
In the present study, only the baseline and peak temperatures were measured while RF was applied. If an assumption is made that the peak temperature attained was maintained for the total duration of treatment for each participant, the mean (± SD) CEM 43 °C thermal dose for the sample can be calculated to be 0.0004 (± 0.0008) min for the CAP mode and 0.0005 (± 0.0008) min for the RES mode. However, this calculation is only applicable to the area of skin from where the temperature measurements were taken. No such estimation can be given using the current data for the deeper tissues, where the thermal dose might have been completely different.
The peak powers reported in and ) do not necessarily provide an accurate reflection of the CRMRF powers required to achieve the three time points in a normal treatment session where a set treatment dose may be delivered from the beginning. Rather, they denote the peak powers that may be achieved if the intensity is incrementally raised every 30 s starting at the minimum dose. The mean (± SD) of mean powers (MP) (the ‘mean overall power’ provided for each participant for the duration of the treatment) delivered to reach the thermal onset, definite thermal and thermal discomfort stages were 3.89 (± 0.99), 6.05 (± 1.36) and 11.70 (± 1.67) W respectively for the CAP mode and 10.61 (± 3.78), 17.87 (± 5.36) and 28.74 (± 4.74) W respectively for the RES mode.
The skin temperatures at the thermal onset and definite thermal stages were not recorded, as this would have affected the continuity of treatment and delayed the attainment of the subsequent thermal stage due to a ‘thermal washout’. The peak skin temperatures attained were not significantly different between the two modes despite the non-linearity of their intensity settings. However, while the CAP mode showed a sharp decline in the (mean) skin temperature in the immediate post-treatment phase, by contrast the RES mode (mean) skin temperature showed an increase in the first few minutes after the treatment ended. These dissimilar thermal output patterns are unlikely to have been influenced by the intensity settings.
More than 60% of the gained heat (Mean (± SD) of 60.3 (± 34.5)%) has been retained in the RES mode at the end of the 45-min follow-up, as against 15% (Mean (± SD) of 15.5 (± 20.1)%) in the CAP mode (). The untreated control side and the core temperatures did not change significantly at any point for either condition. While it was unsurprising that the core temperature did not change since a local application of RF energy is not expected to influence the core temperature [Citation6], the results of the control leg is contrary to the ‘crosstalk’ effect (undesirable effect generated in the neighbouring untreated area) that has been reported in SWT research [Citation35]. This may be due to potentially lower levels of ‘scattering’ of the waves from CRMRF therapy compared to SWT.
The faster heating and faster thermal decay associated with the CAP mode CRMRF may be indicative of its relatively superficial nature of penetration. Similar to the ‘capacitive method’ of application in SWT, there may be a higher proportion of ‘electric’ (E) than ‘magnetic’ (H) field causing a capacitive (faradic) effect in the tissues [Citation4,Citation11]. Proportionally, more energy is absorbed in the superficial tissues (such as the skin and fat layers) than in the deeper tissues (underlying tissues such as muscles) as there is a higher concentration of field in the superficial tissues [Citation36,Citation37].
Similarly, the RES mode CRMRF is comparable to the ‘inductive method’ in SWT as they both generate an effect that is predominantly due to an H field [Citation4,Citation11]. The higher retention of heat and the fact that there was no sharp fall in the post-treatment temperature strongly suggests higher energy penetration. While the energy absorption for inductive treatment is greater in deeper tissues such as blood and muscles due to their lower resistance and higher electrolyte content, tissues such as skin and subcutaneous fat that have higher resistance absorb less energy and therefore heat up less compared to the deeper tissues [Citation11,Citation37].
The amount of heat generated is proportional to the conductivity of the tissues and the strength of the field [Citation4]. Penetration by the RF energy is also influenced by the size of treatment electrodes; with the larger electrodes having more penetrative ability than the smaller electrodes [Citation11]. There is a fundamental difference between SWT and CRMRF when the frequency and other characteristics of their RF waves are considered, hence, all the physical principles of shortwave-tissue interaction may not apply to CRMRF. When compared to SWT, there is little published research on the physical principles of CRMRF-tissue interaction.
The results obtained here are likely to have been influenced by the size (large; 65 mm in diameter) and geometry (circular) of the active electrodes used, the amount of conductive cream left on the skin, and other individual factors such as the anatomy of the area treated, tissue resistance and potentially the anthropometric factors (e.g. body fat percentage, body mass index). However, the crossover study design would have mitigated the influence of such individual factors on the analysis of these results.
The electrode size used was appropriate for the size of the area treated, potentially helping the homogeneity of field distribution. The size of electrode influences the magnitude of electromagnetic field generated and the field/current density [Citation11]. With large electrodes the field density would not have been disproportionately high under the active electrode, thus ensuring a more even distribution of energy. The research surrounding the field/current density is a separate area in itself, which was not designed to be analysed in the present study.
As reported in , the temperatures of the active electrodes at similar intensities of CRMRF application as in the main experiment were not significantly different from the peak skin temperatures reached. The peak RES mode electrode temperature remained consistently lower than that of the skin, while the CAP mode electrode temperature increased marginally when the intensity of exposure was sustained for longer periods. This suggests that the rise in skin temperature was not a direct result of contact heating from a hot electrode, but rather due to RF–tissue interaction.
The participants generally reported a more superficial ‘feel’ for heating in the CAP mode, with the heating felt mainly on the skin of the treated area. Little heat was reported through the knee joint or into the calf. However, the ‘feel’ was reported to be deeper and more homogenous with the RES mode, with the knee joint and calf involved.
However, the claims about perception of heat at depth should be interpreted with caution, because localised thermal perception is based primarily on cutaneous receptors [Citation2] and there remains some controversy as to whether thermal perception at depth is thermal perception per se or nociception. To date there is insufficient evidence on the existence of subjective perception of temperature from deeper tissues such as muscle [Citation38]. There may be a mechanism of thermal perception at depth, but it may be at a subconscious level when tissue temperature is approximately between 25–41 °C. Thermal perception in muscle beyond this level is arguably less potent compared to nociception. The question also remains as to whether the nociceptors and other pressure and mechanosensitive receptors in the muscle might also function as peripheral sensors for subjective temperature sensation in humans [Citation38,Citation39].
The sensation of heat in the tissues depends on the pattern of thermal energy delivery (e.g. thermal change, rate of thermal change) [Citation1,Citation2,Citation6]. For the above reasons it is therefore proposed that although deep heating may have occurred from the CRMRF therapy, the different heating sensations reported from the CAP and RES modes by the participants in this study may relate to a variation in the rate and distribution of temperature change in the more superficial tissues such as the skin and superficial fascia, where there is a presence of thermoreceptors.
In this study any potential variability in the thermal response data between individual participants may be explained by the variability in their anthropometric factors. Although it was not the primary aim of the study, the correlation between the anthropometric factors and the thermal responses was analysed in order to assess whether a prediction of one’s thermal response is possible from their anthropometric and baseline data using a statistical (linear regression) model.
The body fat percentage appeared to have a moderate negative correlation (significant at p < 0.05 level) with the CAP thermal onset and discomfort data, whereas the same was observed only for the thermal discomfort data in the RES mode. A moderate negative correlation (significant at p < 0.05 level) was also observed between the room temperature and the RES mode thermal onset and definite thermal data. No other anthropometric factors correlated with the thermal response data. Hence, an appropriate statistical model for the prediction of one’s thermal response and treatment doses could not be determined for either mode of treatment using the data in this study.
To our knowledge, this is the first study investigating the skin thermal responses to local therapeutic application of RF below the shortwave frequencies. Several studies investigating the skin and/or deep thermal responses of humans resulting from exposure to RF (predominantly the shortwave frequencies) have been identified [Citation35,Citation40–47], although only a limited number of those studies have mapped skin temperature for as long as 45 min post-treatment. A significant rise and maintenance of both skin and intra-muscular temperatures were reported by many of the earlier published studies [Citation40,Citation42,Citation43,Citation47].
Among the more recent (non-invasive) studies that have employed incremental doses of PSWT, Bricknell and Watson [Citation41] reported dose parameters that produced a ‘possible thermal perception’ (Mean ‘mean power (MP)’ of 6.58 (± 3.50) W) and a ‘definite thermal perception’ (Mean MP 10.88 (± 3.32) W) on the skin. A mean temperature increase of 2.10 °C from baseline to the definite thermal stage was reported. In another study, Al-Mandeel and Watson [Citation35] reported a significant rise in skin temperature with high (MP 24 W) and low (MP 3 W) doses of PSWT.
Among the invasive studies, Draper et al. [Citation44] investigated the temperature changes inside the gastrocnemius muscle in response to a 20-min treatment with PSWT (MP 48 W). A mean (± SD) temperature rise of 3.49 (± 1.13) °C was reported after the treatment and a decay of 1.78 (± 0.69) °C was reported in the first 10 min post-treatment. Later, in a non-shortwave study, Takahashi et al. [Citation45] reported a 5 °C rise in temperature inside the knee joint with a 20-min pulsed RF treatment (8 MHz, 200 W) on patients suffering from osteoarthritis of the knee. The temperature decay was not monitored in this study.
A potential limitation of the current study is that it was unable to investigate the temperature changes at depth, despite it being evident from the results that considerable absorption of heat may have occurred in the deeper tissues. While appropriate non-invasive methods for temperature measurement at depth were not available to the authors, several potential issues exist with invasive temperature measurements. For example, where the temperature recording device (thermocouples or thermistors) was left in situ while the RF was delivered (as in the study by Takahashi et al. [Citation45]), it is highly likely that the electromagnetic field might have directly heated the measurement device thus showing a higher temperature recording. Furthermore, the invasive procedure triggers a tissue inflammatory response, which potentially affects the local physiological activity (such as a change in the local blood flow).
Non-invasive temperature measurement at depth using techniques such as magnetic resonance (MR thermometry) and ultrasound (US thermometry) are gaining ground and are employed in areas like cancer thermotherapy [Citation48–51]. Such techniques are far more complex and were beyond the scope of this study. Nonetheless, similar studies employing such comprehensive non-invasive measurement systems to investigate the thermal effects of CRMRF based treatment in deeper tissues should be welcomed in the future.
In addition to deep temperature, the skin temperature at the thermal onset and definite thermal stages were also not recorded in this study. Considering the methodology that was adopted in this experiment, it would have been difficult to record those temperatures without significantly affecting the continuity of treatment.
Likewise, the thermal response patterns reported here may only be applied to the size of active electrodes (65 mm) used in this experiment due to the considerations on electric field density; and the body area treated due to anatomical considerations. The thermal properties of the conductive cream and the amount of cream left on the treated area towards the end of treatment also would have influenced the attainment of thermal stages.
A further limitation commonly found in laboratory studies is that the participants may be young and physically fit subjects, limiting the generalisability of the results. In this study, although the participants were physically fit and active; their age range (19–59 years) was purposefully kept wide to improve generalisability. In any case, extrapolation of these results to a patient population is problematic as their physiological responses will be different due to the underlying pathology.
Conclusions
This study confirms that both the CAP and the RES modes of CRMRF therapy can significantly increase and sustain skin temperature. Average time, energy and power required to achieve thermal onset, definite thermal and thermal discomfort sensations when the doses were delivered incrementally (as described), were identified for both modes of treatment.
This study did not aim to provide recommendations on dosing based on perceived heat, and per se, the results cannot be extrapolated to a patient population. However, they may provide guidance to clinicians for clinical decision-making when they decide to use such therapy. The study also provides useful baseline data for further research in the low frequency ranges of RF therapy that have remained largely unexplored. Further studies that address the limitations of this study and explore additional physiological responses (such as blood flow, temperature at depth), and clinical studies that involve patient groups are therefore necessary.
Acknowledgements
The authors would like to thank all the members of staff and students of the University of Hertfordshire who kindly volunteered to take part in this study and spent several hours of their valuable time in the lab.
Declaration of interest
The University of Hertfordshire is in receipt of an industry-linked research funding related to this programme of research (Indiba S. A.). The industry funders had no role in the study design, data collection, data analysis or the preparation of this manuscript. The authors alone are responsible for the content and writing of the paper.
References
- Watson T. Electrotherapy: Evidence-Based Practice. 12th ed. London: Elsevier Churchill Livingstone; 2008
- Guyton AC, Hall JE. Textbook of Medical Physiology, 12th ed. Philadelphia: Elsevier Saunders; 2011
- Lehmann J, DeLateur B. Therapeutic heat. In: Lehmann J, editor. Therapeutic Heat and Cold, 4th ed. Baltimore: Williams & Wilkins; 1990. pp 470–4
- Prentice W, Draper D. Shortwave and microwave diathermy. In: Prentice W, editor. Therapeutic Modalities in Rehabilitation. 4th ed. New York: McGraw-Hill; 2011. p. 433–62
- Krusen FH. Short wave diathermy in industrial rehabilitation. Am J Surg 1938;42:845–50
- Adair ER, Black DR. Thermoregulatory responses to RF energy absorption. Bioelectromagnetics. 2003;(Suppl6):S17–38
- Valtonen EJ, Alaranta H. Comparative clinical study of the effect of short-wave and long-wave diathermy on osteo-arthritis of the knee and hip. Scand J Rehabil Med 1971;3:109–12
- Shah SGS, Farrow A. Trends in the availability and usage of electrophysical agents in physiotherapy practices from 1990 to 2010: A review. Phys Ther Rev 2012;17:207–26
- Goats GC. Microwave diathermy. Br J Sports Med. 1990;24:212–18
- Kitchen S, Partridge C. Review of shortwave diathermy continuous and pulsed patterns. Physiotherapy 1992;78:243–52
- Al-Mandeel M, Watson T. Pulsed and continuous short wave therapy. In: Watson T, editor. Electrotherapy: Evidence-Based Practice, 12th ed. London: Elsevier Churchill Livingstone; 2008. pp 137–60
- Hayne C. Pulsed frequency energy, its place in physiotherapy. Physiotherapy 1984;70:259–66
- Swicord ML, Balzano Q, Sheppard AR. A review of physical mechanisms of radiofrequency interaction with biological systems. IEEE Asia–Pacific International Symposium on Electromagnetic Compatibility. Beijing: IEEE; 2010. pp 21–4
- Kumaran B, Watson T. Radiofrequency-based treatment in therapy-related clinical practice – a narrative review. Part I: acute conditions. Phys Ther Rev. 2015;20(4):241–54
- Giannakopoulos XK, Giotis C, Karkabounas S, Verginadis, II, Simos YV, Peschos D, Evangelou AM. Effects of pulsed electromagnetic fields on benign prostate hyperplasia. Int Urol Nephrol 2011;43:955–60
- Sakai H, Horiguchi N, Endoh D, Nakayama K, Hayashi M. Radiofrequency radiation at 40 kHz induces hepatic injury in Long-Evans Cinnamon (LEC) rats, an animal model for human Wilson disease. J Vet Med Sci. 2011;73:299–304
- Kato S, Saitoh Y, Miwa N. Repressive effects of a capacitive-resistive electric transfer (CRet) hyperthermic apparatus combined with provitamin C on intracellular lipid-droplets formation in adipocytes. Int J Hyperthermia 2013;29:30–7
- Kato S, Asada R, Kageyama K, Saitoh Y, Miwa N. Anticancer effects of 6-o-palmitoyl-ascorbate combined with a capacitive-resistive electric transfer hyperthermic apparatus as compared with ascorbate in relation to ascorbyl radical generation. Cytotechnology 2011;63:425–35
- Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110:20176–81
- Lodhi IJ, Semenkovich CF. Why we should put clothes on mice. Cell Metab 2009;9:111–12
- Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol 2012;37:654–85
- Challis LJ. Mechanisms for interaction between RF fields and biological tissue. Bioelectromagnetics 2005;(Suppl7):S98–106
- Mitchell SM, Trowbridge CA, Fincher AL, Cramer JT. Effect of diathermy on muscle temperature, electromyography, and mechanomyography. Muscle Nerve 2008;38:992–1004
- Knight CA, Rutledge CR, Cox ME, Acosta M, Hall SJ. Effect of superficial heat, deep heat, and active exercise warm-up on the extensibility of the plantar flexors. Phys Ther 2001;81:1206–14
- Robertson VJ, Ward AR, Jung P. The effect of heat on tissue extensibility: A comparison of deep and superficial heating. Arch Phys Med Rehabil 2005;86:819–25
- Field SB, Morris CC. The relationship between heating time and temperature: Its relevance to clinical hyperthermia. Radiother Oncol 1983;1:179–86
- Hall EJ, Roizin-Towle L. Biological effects of heat. Cancer Res. 1984;4410(Suppl):S4708–13
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 2009;25:3–20
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003;19:267–94
- van Rhoon GC, Samaras T, Yarmolenko PS, Dewhirst MW, Neufeld E, Kuster N. CEM43 degrees C thermal dose thresholds: A potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol 2013;23:2215–27
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787–800
- Hernandez-Bule ML, Cid MA, Trillo MA, Leal J, Ubeda A. Cytostatic response of HepG2 to 0.57 MHz electric currents mediated by changes in cell cycle control proteins. Int J Oncol 2010;37:1399–405
- Hernandez-Bule ML, Roldan E, Matilla J, Trillo MA, Ubeda A. Radiofrequency currents exert cytotoxic effects in NB69 human neuroblastoma cells but not in peripheral blood mononuclear cells. Int J Oncol 2012;41:1251–9
- Hernandez-Bule ML, Trillo MA, Cid MA, Leal J, Ubeda A. In vitro exposure to 0.57-MHz electric currents exerts cytostatic effects in HepG2 human hepatocarcinoma cells. Int J Oncol 2007;30:583–92
- Al-Mandeel MM, Watson T. The thermal and nonthermal effects of high and low doses of pulsed short wave therapy (PSWT). Physiother Res Int 2010;15:199–211
- Low J, Reed A. Electromagnetic fields: shortwave diathermy, pulsed electromagnetic energy and magnetic therapies. In: Low J, Reed A, editors. Electrotherapy Explained: Principles and Practice. London: Butterworth Heinemann; 1990. pp 221–60
- Guy AW. Biophysics of high frequency currents and electromagnetic radiation. In: Lehmann JF, ed. Therapeutic Heat and Cold, 3rd ed. Baltimore: Williams & Wilkins; 1982. pp 199–277
- Graven-Nielsen T, Arendt-Nielsen L, Mense S. Thermosensitivity of muscle: High-intensity thermal stimulation of muscle tissue induces muscle pain in humans. J Physiol 2002;540:647–56
- Graven-Nielsen T, Mense S. The peripheral apparatus of muscle pain: Evidence from animal and human studies. Clin J Pain 2001;17:2–10
- Abramson DI, Bell Y, Rejal H, Tuck S, Jr Burnett C, Fleischer CJ. Changes in blood flow, oxygen uptake and tissue temperatures produced by therapeutic physical agents. II. Effect of short-wave diathermy. Am J Phys Med 1960;39:87–95
- Bricknell R, Watson T. The thermal effects of pulsed shortwave therapy. Br J Ther Rehabil 1995;2:430–4
- Bennett RL, Hines Jr EA, Krusen FH. Effect of short-wave diathermy on the cutaneous temperatures of the feet. Am Heart J 1941;21:490–503
- Flax HJ, Miller RN, Horvath SM. Alterations in peripheral circulation and tissue temperature following local application of short wave diathermy. Arch Phys Med Rehabil 1949;30:630–7
- Draper DO, Knight K, Fujiwara T, Castel JC. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther 1999;29:13–22
- Takahashi K, Kurosaki H, Hashimoto S, Takenouchi K, Kamada T, Nakamura H. The effects of radiofrequency hyperthermia on pain and function in patients with knee osteoarthritis: A preliminary report. J Orthop Sci 2011;16:376–81
- Valtonen EJ, Lilius HG, Svinhufvud U. Effects of three modes of application of short wave diathermy on the cutaneous temperature of the legs. Eura Medicophys 1973;9:49–52
- Verrier M, Falconer K, Crawford JS. A comparison of tissue temperature following two shortwave diathermy techniques. Physiother Can 1977;29:21–5
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008;27:376–90
- van Dongen KW, Verweij MD. A feasibility study for non-invasive thermometry using non-linear ultrasound. Int J Hyperthermia 2011;27:612–24
- Arthur RM, Straube WL, Trobaugh JW, Moros EG. Non-invasive estimation of hyperthermia temperatures with ultrasound. Int J Hyperthermia. 2005;21:589–600
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia 2006;22:255–62
