Abstract
Purpose: Anti-angiogenic agents have shown promise for treating advanced hepatocellular carcinoma (HCC), and the primary mechanism of low-dose metronomic chemotherapy using traditional cytotoxic drugs is anti-angiogenic. This study evaluated the efficacy of metronomic capecitabine and thalidomide after cool-tip radiofrequency ablation (RFA), relative to RFA alone, for treating patients with HCC. Methods and materials: Patients with HCC were randomly apportioned to a test group (n = 22) receiving metronomic chemotherapy with capecitabine and thalidomide after RFA, or a control group (n = 28) receiving RFA only. Serum circulating endothelial cells (CECs) and vascular endothelial growth factor (VEGF) were measured in all patients before and 1 month after RFA treatment. Enhanced computed tomography or ultrasound imaging was performed to evaluate efficacy during 12 months of follow-up. The treatment groups were further stratified as HCC within or outside the Milan criteria for transplantation. Results: One month post-treatment, the tumour response rate (TRR), including complete response and partial response rate, of the test and control groups was statistically similar. At 12 months, the TRR of the test group (68.2%) was significantly higher than that of the control group (35.7%). In the test group, the TRR of patients whose tumour burdens were outside the Milan criteria was significantly higher than that of the control group. One month post-treatment, CECs and VEGF levels of the test group were significantly lower than baseline, while those of the control group were significantly higher. At the end of the 12-month follow-up, there was a progression-free survival (PFS) benefit of 2 months in the test group. Conclusion: Metronomic capecitabine and thalidomide after RFA significantly reduced recurrence of HCC and extended PFS, especially for HCC outside the Milan criteria, perhaps via reduction of serum CECs and VEGF levels and inhibition of tumour angiogenesis.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequently diagnosed malignancy worldwide. HCC morbidity is especially high in China, where it is the first and second most common cause of cancer deaths in rural and urban areas, respectively [Citation1,Citation2]. Only 20% of HCC patients are amenable to curative therapy (i.e. liver transplantation or surgical resection), and for the remainder radiofrequency ablation (RFA) is the major treatment [Citation3]. However, recent reports have raised concerns that incomplete RFA of HCC may promote angiogenesis of the residue tumour and lead to recurrence [Citation4,Citation5].
Metronomic chemotherapy is the frequent and long-term administration of low doses of anti-tumour drugs (one tenth to one third of the maximum tolerated dose), with the intention of preventing tumour angiogenesis. Often these drugs are the traditional cytotoxic chemotherapy medicines. Research has shown that metronomic chemotherapy can minimise adverse drug reactions while also targeting both endothelial cells and tumour cells that are at the proliferation stage [Citation6]. The method is well suited for complementary therapy after radical surgical resection and RFA, or as maintenance therapy after disease remission. Currently, few studies have focused on such therapy.
Studies have shown that vascular endothelial growth factor (VEGF) and circulating endothelial cells (CECs) can be used as biomarkers of anti-angiogenic treatment [Citation7,Citation8]. In the present study we investigated the clinical efficacy of metronomic chemotherapy administered after cool-tip radiofrequency ablation (RFA) in the treatment of HCC. We also conducted a preliminary analysis of the mechanism underlying the associated anti-angiogenic effect by measuring serum CECs and VEGF.
Materials and methods
This study was conducted in accordance with the edicts of the Declaration of Helsinki, and was approved by the Ethics Committee of the Affiliated Hospital of Hainan Medical College. Informed consent was obtained from all patients.
Criteria for patient selection
For inclusion in this study all patients conformed to the following criteria [Citation9]: clinically or pathologically diagnosed HCC, tumours falling within the Milan criteria (solitary tumour ≤ 5 cm in diameter, and up to 3 nodules ≤ 3 cm in diameter); and liver function graded as Child-Pugh A or B, or achieving this standard after medical treatment. For patients with tumours that were outside the Milan criteria, local RFA was used as palliative treatment or part of combined therapy such as transcatheter arterial chemoembolisation (TACE).
Patients were excluded who had an excessively large tumour (more than 70% of the liver volume) or diffuse liver cancer, vascular thrombosis or invasion to adjacent organs, Child-Pugh C liver function that was not improved by medical treatment, oesophageal (gastric fundus) variceal bleeding within a month before treatment, uncorrectable coagulation dysfunction, or severely abnormal haemogram indicating high bleeding tendency, intractable massive ascites and cachexia, active infection, especially inflammation of the biliary system; Eastern Cooperative Oncology Group (ECOG) performance status >2, or disturbance of consciousness or other factors that impeded cooperation with medical treatment.
Characteristics of patients
From June 2010 to December 2013, 50 patients with HCC who satisfied the above criteria were recruited in the Department of Medical Oncology, Tumour Institute, at the Affiliated Hospital of Hainan Medical College.
The patients were randomly apportioned to a test group to receive metronomic chemotherapy starting 1 week after RFA, or a control group to receive RFA only. The test group (n = 22) consisted of 14 men and 8 women; aged 39–75 years (median age, 59 years); 12 patients were naive to surgery and 10 patients had tumour recurrence after surgery. The largest tumour was 3.8 ± 2.6 cm (range 3.1–8.4 cm) in diameter; 10 patients were Child-Pugh A and 12 patients were Child-Pugh B, 13 patients had tumours within the Milan criteria for transplantation and 9 patients had tumours outside the criteria.
The control group (n = 28) comprised 17 men and 11 women; aged 36–73 years (median age, 58 years); 14 were naive to surgery and 14 recurred after surgery. The largest tumour was 4.0 ± 2.8 cm (range 2.6–9.0 cm) in diameter; 13 patients were Child-Pugh A and 15 were Child-Pugh B, 16 patients had tumours within the Milan criteria and 12 patients had tumours outside the criteria.
Statistical analyses showed no significant difference in gender ratios, age, tumour size, or liver function between the two groups (p > 0.05).
Equipment and reagents
A RFTI-1 cool-tip RFA apparatus was used (Nanjing Tianma High-tech), which included a radiofrequency source, a peristaltic pump, cool-tip electrode, and neutral electrode plate. Puncture was guided using a Philips HD11 ultrasound system or Siemens 16-slice spiral computed tomography (CT). The FACSCalibur flow cytometer, Percp-CD45 antibody, allophycocyanin (APC)-CD133 antibody, phycoerythrin (PE)-CD146 antibody, and control antibody were purchased from BD Biosciences, San Jose, USA. The human VEGF enzyme-linked immunosorbent assay (ELISA) kit was purchased from Dingguochangsheng Biotechnology (Beijing, China).
RFA protocol
Preoperative imaging was performed to assess tumour size, location, number, edge, blood supply, and adjacent organs of all patients. All the participants of the study underwent the RFA procedure. The patients were fasted for 6 h before surgery, and were given intramuscular (IM) 50 mg pethidine and 25 mg Phenergan 15 min before surgery. During the surgery, patients were given intravenous 300 mg tramadol to relieve pain, and 2% lidocaine for local anaesthesia. Electrocardiographic monitoring was performed during the surgery and rescue supplies were prepared within reach.
Lesions <3 cm in diameter were ablated with a single puncture. Lesions > 3 cm were ablated by multiple punctures, with the centre ablated first and the peripheral afterward. Ablation was intended to cover the entire tumour and >1.0 cm beyond the edge, to maximise damage to the lesion. After positioning the electrode under the guidance of coloured ultrasound or CT, the cool-tip pump was opened and the impedance mode was chosen for RFA treatment. The RF output of the therapeutic apparatus is determined by the impedance. When the impedance is more than the original 10 Ω, the radio frequency current is reduced to 0.1 A, staying there for a few seconds, continuing to increase the output power, and the cycle was repeated. Treatment time was set to 12 min for each site, and additional treatment was performed by multiple punctures, depending on the condition of the lesions. Before withdrawal of the needle, the system was set to manual mode at 35 W for ablation of the needle tract.
TACE procedure
The right femoral artery was punctured using the Seldinger technique after local anaesthesia. According to the findings of angiography, a micro-catheter was super-selectively catheterised into the tumour-feeding artery and the chemoembolisation was conducted through this catheter. The type and dose of chemotherapeutic agents, including hydroxycamptothecine (10–30 mg) and fluorouracil (500–1000 mg), were determined by the tumour condition, liver and kidney function. After drug administrations, lipiodol-cisplatin was used to block the vessels. The RFA procedure was performed after 2 weeks of TACE.
Metronomic chemotherapy
Capecitabine tablets (Roche Pharmaceuticals, Shanghai, batch number: H20073024), 0.5 g twice per day, and thalidomide tablets (Changzhou Pharmaceutical, batch number: H32026129), 50 mg twice per day, were given to patients in the test group until tumour progression or the side effects became intolerable.
Laboratory measurement of VEGF and CECs
Peripheral blood of the patients was sampled 1 month before and after RFA treatment. Serum VEGF levels were detected by ELISA. CECs (cluster of differentiation CD45-CD133-CD146+) were labelled with fluorescent antibody and counted using flow cytometry and CELL Quest software [Citation10].
Efficacy assessment of RFA
Enhanced CT or ultrasound imaging was performed 1 month after RFA treatment to assess the efficacy of RFA. According to expert consensus on the norms of local ablation therapy for HCC [Citation9], tumour response was classified as complete or partial. A complete response was considered complete ablation, with triple-phase CT indicating low-density of the original tumour area, or high echo in ultrasound imaging without enhancement in the arterial phase during the follow-up. A partial response included incomplete ablation, triple-phase CT showing residue of the tumour, or an ultrasound indicating enhancement in the arterial phase during the follow-up.
For tumour residue, a second treatment, including RFA, TACE or RFA + TACE, was performed, but if the second treatment failed to eradicate the tumour, alternative treatment was applied. The following events were considered tumour progression: local tumour progression (new tumours emerging adjacent to the original tumour after RFA); new lesion (new tumours emerging in the liver away from the original tumour after RFA); and distant metastasis (new tumours emerging away from the liver). When tumours progress, a second RFA or alternative treatment should be implemented. Adverse reactions after metronomic chemotherapy were graded into 1–5 levels in accordance with the National Cancer Institute CTC version 4.0 standard.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 statistical software. CECs and VEGF levels conformed to normal distributions indicated by exploratory analysis, and these were analysed by group and paired t tests. Rates of response and progression were analysed using chi-square tests. Progression-free survival was analysed by Kaplan-Meier assay, and differences between the two groups were analysed with the log-rank test. p < 0.05 was considered statistically significant.
Results
Efficacy and adverse reactions
A total of 80 electrode needles were used in the 50 patients, including four cluster needles.
After 1 month of treatment, the efficacy of the test and control groups was statistically similar (χ2 = 0.064, p = 0.801; see ). Specifically, as indicated by enhanced CT or ultrasound examination, the tumour response rate (TRR) of the test group was 90.9% (20/22; 14 and six cases of complete and partial response, respectively), and the tumour progression rate (TPR) was 9.1% (2/22) with two cases of local tumour progression. The TRR of the control group was 92.9% (26/28; 17 and nine cases of complete and partial response, respectively), and the TPR was 7.1% (2/28) with one case of local tumour progression and one new lesion. In the test group there were six cases of partial response, two cases were treated with the second RFA alone, and three cases combined RFA with TACE. In the control group there were nine cases of partial response, two cases were treated with the second RFA alone, and five cases combined RFA with TACE.
Table 1. Efficacy of HCC, numbers of casesa.
During 12 months of follow-up, the difference in the efficacy between the two groups was significant (χ2 = 5.915, p = 0.023; see ). Specifically, patients in the test group experienced a TRR of 68.2% (15/22) and a TPR of 31.8% (7/22) with two, three, and two cases of local tumour progression, new lesions, and distant metastasis, respectively. The control group had a TRR of 35.7% (10/28) and a TPR of 64.3% (18/28), with four, five, and nine cases of local tumour progression, new lesions, and distant metastasis, respectively.
The subgroup analysis of patients with HCC that were within the Milan criteria showed no statistical difference in the efficacy between the test and control groups (χ2 = 0.697, p = 0.404; see ). Specifically, for patients in the test group with HCC that conformed to the Milan criteria, the TRR was 76.9% (10/13) and the TPR was 23.1% (3/13), with two and one case of local tumour progression and new lesion, respectively. For patients in the control group with HCC that were within the Milan criteria, the TRR was 62.5% (10/16) and the TPR was 37.5% (6/16), with two, two, and two cases of local tumour progression, new lesion, and distant metastasis, respectively.
The subgroup analysis of patients with HCC that were outside the Milan criteria revealed a significant difference in the efficacy between the test and control groups (χ2 = 8.750, p = 0.003; see ). Specifically, for patients in the test group with HCC that were outside the Milan criteria, the TRR was 55.6% (5/9) and the TPR was 44.4% (4/9), with one, one, and two cases of local tumour progression, new lesion, and distant metastasis, respectively. For patients in the control group with HCC that were outside the Milan criteria, the TRR was nil (0/12) and the TPR was 100% (12/12), with three, three, and six cases of local tumour progression, new lesion, and distant metastasis, respectively.
None of the 50 HCC patients in this study died during surgery. All the patients felt varying degrees of pain due to heat in the area of the liver. Ten experienced nausea and vomiting, and six reported chest tightness and discomfort in the upper right abdomen. No pneumothorax, perforation in the stomach, intestine, or gallbladder, or major bleeding occurred during surgery. Sixteen of the cases presented with fever of 37.8–39.8 °C after surgery, with an average duration of 3 days. No liver abscesses or needle-tract metastasis occurred after surgery. Adverse reactions due to metronomic chemotherapy were mild, mainly manifested by Grade 1 hand-foot syndrome (four cases) and impairment of liver function (five cases of Grade 1, 3 cases of Grade 2, and no cases of Grades 3–5).
Changes in serum CECs and VEGF levels
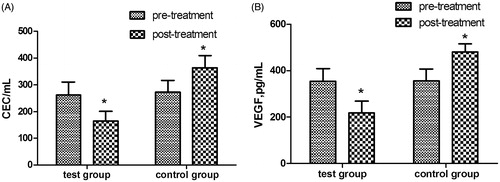
After RFA treatment for 1 month, serum CECs and VEGF levels in the test group were significantly lower than they were before treatment (baseline), and also significantly lower than those of the control group at the same time point (p < 0.05, see ). However, the serum CECs and VEGF levels of the control group were significantly higher than they were at baseline (p < 0.05).
Progression-free survival analysis
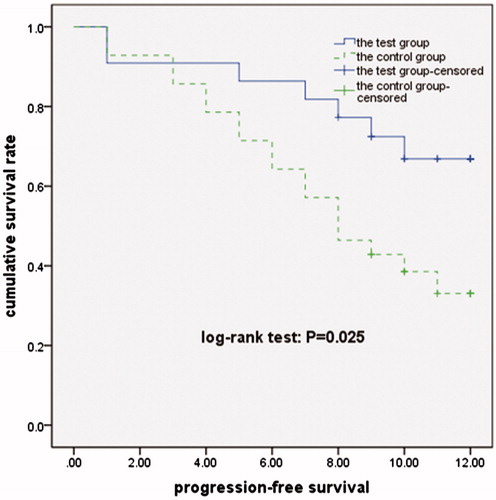
At the end of the 12-month follow-up, progression-free survival (PFS) of the test and control group was 10.02 and 8.02 months respectively, PFS of the test group was extended by 2.00 months (χ2 = 4.990, p = 0.025, see ).
Discussion
In recent years, RFA has been widely used around the world because it is safe – it can be performed multiple times with little adverse effect or pain while achieving good efficacy. Studies have shown that RFA achieves the best efficacy for HCCs that are less than 3 cm in diameter, with a 5-year survival rate that is comparable to that of partial liver resection [Citation11]. With advancements in technology, the third generation of optimised electrodes (i.e. the clustered internal-cooling electrode and the saline-enhanced umbrella-like composite electrode) greatly extended the application range of RFA in all stages of HCC, including HCC beyond the Milan criteria for transplantation. In this study we used the new generation cool-tip RFA instrument, which cools the electrode with circulating fluid. Thus heating of the electrode is prevented and carbonisation of the surrounding tissue is avoided. The total energy delivered can be increased and the thermal coagulation effect can be enhanced to maximise the ablation effect. However, for RFA alone for treatment of HCC that were outside the Milan criteria, the TRR was up to 76.2% (16/21) at the end of the 12-month follow-up. Research has shown that RFA + TACE had advantages in improving TRR while the difference was not significant between RFA + TACE and RFA alone on efficacy for small HCC [Citation12].Therefore, in this study some patients with partial response were treated with the second RFA + TACE(three cases in the test group and five in the control group).
With the popularisation of RFA treatment, clinical studies have shown that the rate of local recurrence ranges from 2–53%, and the rate of distant recurrence is 43–53% [Citation13,Citation14]. The main reason for recurrence may be incomplete ablation, which allows the relapse of residue tumour tissue. The reasons for incomplete ablation include limitations associated with anatomical location of the tumour [Citation15], heat loss via tumour blood vessels, the presence of a low-temperature area in superimposed ablation for a large tumour, and the difference during the RFA procedure [Citation16].Tumour relapse after incomplete ablation may be caused, at least partially, by the altered microenvironment after RFA. The alteration may promote angiogenesis via related endothelial cells, enhance invasion of residue tumour cells [Citation17], and increase VEGF and fibroblast growth factor-2(FGF-2) expression by residue tumour cells in the ablation area, allowing the growth of micro-metastases [Citation5]. In this study, serum CECs and VEGF levels of the control group were elevated after RFA, and TRR reached 64.3%. It is suspected that incomplete ablation elevated CECs and VEGF levels and thus promoted metastasis of the residue tumour cells. Therefore, tumour angiogenesis has an important role in recurrence of HCC after RFA. Microvascular invasion also can predict the post-operative recurrence of HCC [Citation18].
Metronomic chemotherapy is also known as anti-angiogenesis chemotherapy. While traditional chemotherapy is delivered to eliminate the tumour, the main purpose of metronomic chemotherapy is to inhibit tumour angiogenesis. An advantage of metronomic chemotherapy over the traditional method is that it targets genetically stable endothelial cells. Thus, it is thought that there is less chance of developing acquired drug resistance. It also prevents intermittent tumour vascular repair that is associated with the intervals between conventional maximum tolerated dose (MTD) chemotherapies, by inhibiting or destroying bone marrow-derived CECs. Metronomic chemotherapy also restores anti-tumour immunity by reducing regulatory T cells, and avoids or reduces the adverse side effects commonly seen in traditional MTD chemotherapy [Citation19–21].
Clinical trials of metronomic chemotherapy have included both chemotherapeutic and anti-angiogenic agents. Metronomic capecitabine was used in many tumours [Citation22,Citation23], while thalidomide is considered a classic anti-angiogenic drug [Citation24]. Recently, various anti-angiogenesis agents have shown promising results in patients with HCC [Citation25,Citation26]. The present study tested the efficacy of metronomic chemotherapy using capecitabine and thalidomide after RFA, and the results indicate that such therapy significantly reduced serum CECs and VEGF levels of these HCC patients, compared with RFA alone. After 1 year of follow-up, the TPR of the test group was significantly lower than that of the control group. Further analysis indicated that progression-free survival of the test group was 2.00 months longer than that of the control group. Currently there is no promising systemic chemotherapy, immunological, or hormonal therapy for HCC, and sorafenib is the only approved therapy for cases outside the Milan criteria [Citation27]. It is noteworthy that patients of the test group with HCC that were outside the Milan criteria had a TRR that was significantly higher than that of corresponding patients in the control group during 12 months of follow-up. In addition, adverse reactions of the metronomic therapy were mild and well tolerated, and no patients withdrew from treatment due to adverse effects.
However, overall survival was not analysed in this study due to the lack of long-term follow-up, and serum CECs and VEGF levels after RFA treatment were accessed at only a few time points. The link between CECs and VEGF levels and tumour progression cannot be determined, and is worthy of further investigation.
In summary, metronomic chemotherapy with capecitabine and thalidomide after RFA was associated with a significant reduction in the tumour recurrence rate and longer progression-free survival in HCC patients. These results were especially noteworthy for HCC that were outside the Milan criteria for transplantation. The efficacy of metronomic capecitabine and thalidomide observed in this study may be related to a reduction in serum CECs and VEGF levels, and to the inhibition of tumour angiogenesis.
Acknowledgements
W.Y.Z. and J.Z.Z. contributed equally to this article.
Declaration of interest
We declare that we have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917
- Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: A review of epidemiology and control measures. J Epidemiol 2011;21:401–16
- Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia 2013;29:558–68
- Kong J, Kong J, Pan B, Ke S, Dong S, Li X, et al. Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF-1alpha/VEGFA. PLoS One 2012;7:e37266
- Kong J, Kong L, Kong J, Ke S, Gao J, Ding X, et al. After insufficient radiofrequency ablation, tumor-associated endothelial cells exhibit enhanced angiogenesis and promote invasiveness of residual hepatocellular carcinoma. J Transl Med 2012;10:230
- Maiti R. Metronomic chemotherapy. J Pharmacol Pharmacother 2014;5:186–92
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–41
- Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat Rev Cancer 2006;6:835–45
- Chinese Society of Liver Cancer CA-CA, Chinese Society of Clinical Oncology CA-CA, Liver Cancer Study Group CSoHCMA. Expert consensus on the norms of local ablation therapy for hepatocellular carcinoma. Zhonghua Gan Zang Bing Za Zhi 2011;19:257–9
- Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 2006;108:452–9
- Zhang L, Ge NL, Chen Y, Xie XY, Yin X, Gan YH, et al. Long-term outcomes and prognostic analysis of radiofrequency ablation for small hepatocellular carcinoma: 10-year follow-up in Chinese patients. Med Oncol 2015;32:77–86
- Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2013;24:3872–82
- Kim H, Rhim H, Choi D, Lim HK, Kim YS, Lee WJ, et al. Recurrence and treatment pattern in long-term survivors with hepatocellular carcinoma: A comparison between radiofrequency ablation and surgery as a first-line treatment. World J Surg 2010;34:1881–6
- Livraghi T. Radiofrequency ablation of hepatocellular carcinoma. Surg Oncol Clin N Am 2011;20:281–99
- Tang T, Feng X, Yan J, Xia F, Li X, Ma K, et al. Predictive value of indocyanine green retention rate with respect to complications of radiofrequency ablation in 878 patients with hepatocellular carcinoma. Int J Hyperthermia 2014;30:402–7
- Zhang B, Moser MA, Zhang EM, Luo Y, Zhang H, Zhang W. Study of the relationship between the target tissue necrosis volume and the target tissue size in liver tumours using two-compartment finite element RFA modelling. Int J Hyperthermia 2014;30:593–602
- Obara K, Matsumoto N, Okamoto M, Kobayashi M, Ikeda H, Takahashi H, et al. Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hepatol Int 2008;2:116–23
- Hung HH, Lei HJ, Chau GY, Su CW, Hsia CY, Kao WY, et al. Milan criteria, multi-nodularity, and microvascular invasion predict the recurrence patterns of hepatocellular carcinoma after resection. J Gastrointest Surg 2013;4:702–11
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 2004;4:423–36
- Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther 2011;19:1737–46
- Pasquier E, Kieran MW, Sterba J, Shaked Y, Baruchel S, Oberlin O, et al. Moving forward with metronomic chemotherapy: Meeting report of the 2nd International Workshop on Metronomic and Anti-Angiogenic Chemotherapy in Paediatric Oncology. Transl Oncol 2011;4:203–11
- Alagizy HA, Shehata MA, Hashem TA, Abdelaziz KK, Swiha MM. Metronomic capecitabine as extended adjuvant chemotherapy in women with triple negative breast cancer. Hematol Oncol Stem Cell Ther 2014;8:22–7
- Yuan F, Shi H, Ji J, Cai Q, Chen X, Yu Y, et al. Capecitabine metronomic chemotherapy inhibits the proliferation of gastric cancer cells through anti-angiogenesis. Oncol Rep 2015;33:1753–62
- Martinez-Frias ML. The thalidomide experience: review of its effects 50 years later. Med Clin (Barc) 2012;139:25–32
- Fang P, Hu JH, Cheng ZG, Liu ZF, Wang JL, Jiao SC. Efficacy and safety of bevacizumab for the treatment of advanced hepatocellular carcinoma: A systematic review of phase II trials. PLoS One 2012;7:e49717
- Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: A phase II study. Oncologist 2013;18:1256–7
- Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol 2015;4:673–87