Abstract
Purpose: We describe the design and application of a new apparatus for applying Radiofrequency (RF) electromagnetic fields to cells in culture on a microscope stage. This new design enables real-time studies of the actuation of magnetic nanoparticles bound to membrane receptors or internalised within cells together with the study of magnetic fluid hyperthermia (MFH)-associated effects. Materials and methods: RF coils were fabricated and electromagnetic simulations were performed along with compatibility evaluations and calorimetric experiments using this apparatus at discreet frequencies between 100 kHz and 1 MHz. Cell killing via MFH was investigated in a neuroblastoma tumour cell line. Results: Simulations and evaluations showed that the field intensity and homogeneity experienced by the cells within the chamber is best with a planar coil configuration. The incubation chamber was suitable for cell culture and the design was compatible with mountings on different makes of microscopes as it mimics a standard 96/24/6 tissue-culture well plate. Successful calorimetric and MFH cytotoxicity proof-of-principle experiments were performed and are presented. Conclusions: We conclude from these experiments that alternating magnetic field (AMF)-mediated activation and magnetic fluid hyperthermia (MFH) research will benefit from this RF coil that fits inside an incubation chamber, mounted onto a microscope. This new design could be used to assist real-time MFH studies in vitro.
Introduction
Established methods of treating cancerous cells have involved such techniques as using chemotherapy and radiotherapy. More recently, advances have been made using magnetic nanoparticles (MNPs), in particular involving the application of radiofrequency (RF) fields to MNPs which are bound to receptors on cell membranes or internalised within cells. This application has been used to facilitate the activation of chemical signalling in the cell leading to cell death, or to deposit energy, triggering necrosis (cell death or tissue damage in an organ) or apoptosis (programmed cell death) [Citation1,Citation2,Citation3,Citation4]. An apparatus designed for observing and monitoring cell responses under a microscope in real-time would clearly benefit this research. In this paper we demonstrate a novel design that provides RF fields on a microscopic stage. It is well known that cell surface receptors respond to external signals such as binding to extracellular molecules, changes in electric potential across the membrane, localised heating or mechanical stimulation, which result in the activation of certain biochemical cell signalling pathways. These pathways control functions such as cell division, apoptosis, matrix production and membrane transport. Recent work has demonstrated that the application of RF fields to MNPs bound to membrane receptors can activate signalling pathways involved in apoptosis and promote endosomal escape of internalised particles [Citation1]. In addition, RF fields have the ability to couple to MNPs targeted to tumours, delivering energy in the form of heat that has the potential to kill tumour cells or sensitise them prior to radiotherapy – a technique known as magnetic fluid hyperthermia (MFH) [Citation3,Citation5,Citation6]. Thermal losses of MNPs and their suitability for MFH cancer treatment have been researched in depth over the years. Undeniably, there is tremendous scope for developing newer technologies for clinical applications. Instrumentation designed to maintain cells in a healthy state, by providing control over physiological temperature and gas concentrations, while simultaneously applying RF fields for time lapse imaging via optical/fluorescence microscopy will facilitate our understanding of fundamental cell-level processes involved in MFH.
Herein, we discuss the development of an AMF nanomagnetic actuation device for time-lapse imaging of in vitro MNP/cell studies. This device applies AMF for in vitro MNP experiments on cells during real-time microscopy in order to ascertain their suitability and to understand the mechanisms at work in magnetic fluid hyperthermia and other AMF mediated nanomagnetic actuation research. We engineered two different instrument designs, each being evaluated for their suitability for maximum field intensity at the sample, in vitro experiment capability, minimisation of stray fields, Petri plate access and positioning.
Methods and materials
Coil set-up
Hollow, water-cooled coils were fabricated with 18 standard wire gauge (SWG) copper wire (Maplin, Stoke on Trent, UK). The outer diameter of the cooling tube was 4.8 mm (Tom Parker, Preston, UK). We were able to accommodate a four-turn solenoid coil or a six-, eight-turn planar coil within the working frame. The geometry of the coils was retained by PVC walls. The dielectric constant (electrical insulation) property for PVC is high, thus it acts as a dielectric shield between the coil and the sample chamber. Furthermore, water runs around the copper wire within the cooling tube, hence convection reduces the non-specific heating from the coil significantly or to nil. A Rogowski current waveform transducer CWT3B/4/100M/5 (Power Electronic Measurements, Nottingham, UK) was used to measure the real-time current flow through the coil in the 100 kHz to 1 MHz frequency range.
Cell incubation chamber
The cell incubation chamber was fabricated with the dimensions of a widely used cell culture plate (Corning, Birmingham, UK) i.e. 127 × 85 × 26 mm, so that the chamber can be mounted onto any type of microscope that accommodates a standard tissue culture plate. A 5-axis computer numerical control (CNC) router (Matsuura, Solihull, UK) was used for precision engineering. The whole chamber was encased within PVC. The set-up was fabricated with a water jacket around the Petri plate chamber and provided with an inlet and an outlet for recirculating water bath connections (Grant Instruments, Shepreth, UK). To cater for biological gas flow another inlet that vents into the Petri plate chamber was incorporated into the system.
AMF instrumentation
Alternating current (AC) magnetic fields were generated by a magneTherm RF field generator (nanoTherics, Stoke-on-Trent, UK) which uses a half bridge circuit, a variable capacitor and a coil to produce frequencies ranging between 1 kHz to 1.2 MHz. The magneTherm standard coil was replaced with the solenoid or planar coil attached to the set-up to achieve frequencies between 100 kHz to 1 MHz with amplitude variations.
Numerical model and flux density calculation
The magnetic flux density (B) simulations were performed using SEMCAD X (SPEAG, Schmid and Partner Engineering, Zurich, Switzerland) This program was extended and developed into the Sim4Life platform (ZMT Zurich MedTech, Zurich). In order to model magnetic flux density (B) distribution from the coils i.e. four-turn solenoid coil together with six-turn and eight-turn planar coil, we have applied the Low Frequency Magneto- Quasi Static (LF M-QS) algorithm via the Finite Element Method model with Dirichlet boundary conditions. In our case the coil wire was treated as a perfect electric conductor (PEC). Coil geometry used for numerical modelling is shown in .
Table 1. Coil geometry and material specification.
One can analytically calculate the magnetic flux (B) from the planar coil using the following formula derived from Biot–Savart's law.
(1)
where µ0 = 4π 10−7 (H/m) is the permeability of free space, N is the number of turns, r is the radius of the planar coil, where log is the natural logarithm, h is the distance from the middle of the coil to the observer along z-axis in our case.
One can also estimate the magnetic field strength (H) and the magnetic flux density (B = µ0H) along the z axis in a finite solenoid using equation (Equation2(2) ).
(2)
where K0 = NIRMS/L and N is the number of coil turns, IRMS is the root mean square value of the current in the coil, L is the length of the coil and a is the radius of the coil [Citation7].
Calorimetric experiment
Aqueous suspension of meso-2,3-dimercaptosuccinic acid (DMSA) stabilised, 15.2 nm sized 10 mg/mL Hypermag™ (nanoTherics, Stoke-on-Trent) was used for calorimetric experiments. ddH2O was used as control. A Pico M with OTG-MPK 5 optical sensor system (Opsens, Quebec, Canada) was used for real-time temperature monitoring, while subjecting the sample/control to AMF. Specific absorption rate (SAR) provides the power generated (watts) per unit mass (gram) of particles in the suspension and was calculated using equation (Equation3(3) ).
(3)
where C is the specific heat capacity of the nanoparticle suspension (J/KmL), φ is the concentration of particles (mg/mL), and ΔT/Δt is the rate of change of temperature over time. Corrected slope method was used to ensure that appropriate region of the data are used for calculations [Citation8]. Measurements were made in the same type of sample container.
Cell culture
SH-SY5Y neuroblastoma cells (from CRL-2266, American Type Culture Collection, Manassas, VA, USA) were seeded in 1000 µL medium onto tissue culture treated 35 mm2 Petri plates (Corning, Birmingham, UK) in Ham’s F12: MEM (1:1) supplemented with 10% FBS, 2 mM L-glutamine, 5 mL 100 U penicillin and 0.1 mg/mL streptomycin. Cells were incubated at 37°C, 5% CO2 (cell culture reagents from Sigma, Gillingham, Dorset, UK).
Cytotoxicity experiment
SH-SY5Y neuroblastoma cells were exposed to 218.7 kHz; 6.56 mT AMF (H × f = 1.14 × 109 Am−1 Hz) for 30 min with 0.48 mg/mL, 0.91 mg/mL, 2 mg/mL concentrations of Hypermag™ particles using the eight-turn planar coil set-up. MNPs were replaced post-30 min exposure with fresh medium. After 24 h incubation the cells were stained with Hoechst stain (Sigma) and propidium iodide (Sigma) and analysed by microscopy using a fluorescent microscope and propidium iodide assay (Leica, Newcastle Upon Tyne, UK). Cytotoxicity was quantified by superimposing fluorescent microscopy images of PI-stained cells and Hoechst-stained cells.
Statistics
Analysis of the significance of the differences between groups of data was performed by one-way ANOVA followed by Bonferroni multiple comparison test; p > 0.05 was considered as not significant.
Microscopy
To test the compatibility of the design with different types of microscopes, the system was mounted onto inverted, upright fluorescence and optical microscopes i.e. Leica, Olympus (Southend-on-Sea, UK), Nikon (Kingston upon Thames, UK), Carl Zeiss, (Cambridge, UK), and Brunei microscopes (Chippenham, UK).
The AMF-mediated nanomagnetic actuation system for time-lapse imaging has dimensions of 127 × 85 × 26 mm. These dimensions were chosen as the system will mimic a widely used cell culture plate format and are compatible with different types of microscope. This system can accommodate 35 mm2 tissue-culture treated Petri plates in which primary cells, immortalised cell lines, tissue slices, tissues can be maintained. A 35 mm2 tissue-culture treated Petri plate with SH-SY5Y neuroblastoma cells taken from a standard CO2 incubator was placed within the Petri plate chamber. Within the sample chamber, temperature was maintained at 37 °C (physiological temperature) with the aid of a water jacket. The water jacket encasing the Petri plate chamber was connected to a recirculating water bath, with circulating water temperature at 40 °C. +3 °C was set in the water bath temperature in order to compensate for conductive heat losses as water flows through the recirculating pump and the tubes. Carbon dioxide input into the Petri plate chamber provisioned with the gas flow vent enabled the cells to constantly receive 5% CO2.
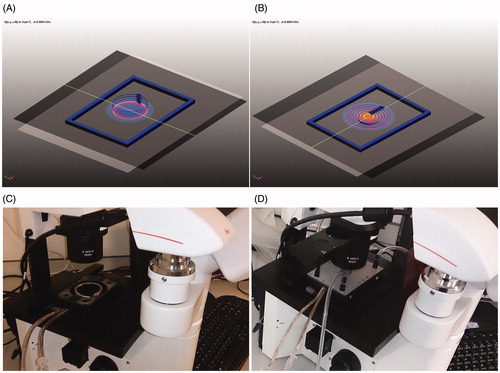
A solenoid was wound around the Petri plate chamber and a planar coil was placed above the chamber to enable AMF exposure. The numbers of turns of the coils were dependent on the available space within the working frame of 127 × 85 × 26 mm, excluding the water jacket dimensions. As the Petri plate can form fit within the chamber on the slot, this also enabled micromanipulation with the aid of the microscope stage (Pecon, Newcastle Upon Tyne) which is usually used for manipulating a cell culture plate.
shows the computer-aided design (CAD) model of the solenoid coil set-up and planar coil set-up with the appropriate turns. The stray field had negligible effect on the adjacent mounting clamps, base plate and optic lenses of the microscope because of positioning of the coil within the PVC wall. The PVC wall also maintains the coil geometry. shows the prototypes mounted on the microscope: silicone with Monel wire RF shield gasket (1.5 mm thick, Laird, St. Louis, Missouri, USA). The two different coil set-ups were attached to the magneTherm. Using variable capacitance within magneTherm and based on the inductance of coils, various AMF frequencies between 100 kHz and 1 MHz were achieved. The same are listed in .
Figure 1. Design of the nanomagnetic AMF actuation set-up for time lapse imaging set-up with the dimensions alike for a cell culture 6, 24, 96 well plate, solenoid coil (A) and planar coil (B). (C, D) Coil systems mounted onto microscope attached with an imaging camera and solenoid coil (C), and planar coil (D).
The Rogowski transducer measurements in the planar coil ranged between 57 IRMS for the lowest frequency and 36 IRMS for the highest frequency, as the resistance in the coil is directly proportional to resonant frequency. In order to obtain a comparison of solenoid coil and planar coil flux density patterns, 45 IRMS and 20 IRMS current flows were simulated.
Results
The results of our experiments are shown in . reveals that the planar coil set-up provides better field amplitude based on our simulations. The contour plot and the line diagram shows that the flux density and homogeneity experienced by the cells within the chamber is better in the planar coil set-up. Therefore, the planar coil was found to be the best fit with our design that is specific to standard cell culture plate dimensions.
Figure 2. Magnetic flux density magneto static simulation for four-turn solenoid zx plane (A) and 6-turn planar zx plane (B) coil at 45 A current rms. (C) Magnetic flux density z axis line diagram for 4-turn solenoid, six-turn planar at 20 A and 45 A current rms.
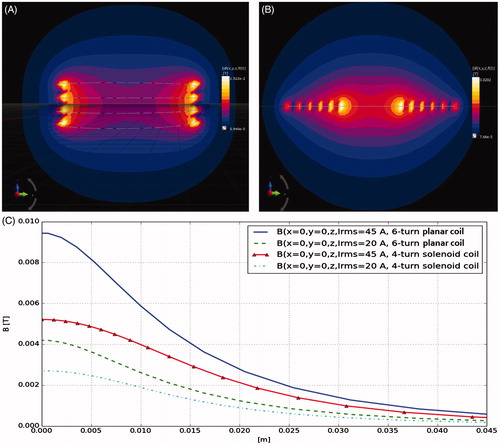
Figure 3. Calorimetry experiments performed using the nanomagnetic AMF actuation planar coil set-up. SAR measurements as a function of frequency at a constant flux density (5 mT). (A) Change of temperature over time graph with water controls and (B) SAR graph.
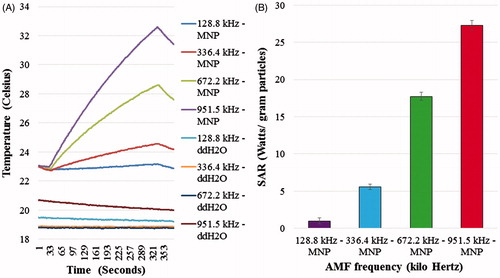
Figure 4. (A) Cell growth media temperature in a Petri plate positioned within the sample chamber for 30 min. (B) Results for 37 °C temperature maintained in a Petri plate with cell growth media positioned within the sample chamber for 30 min.
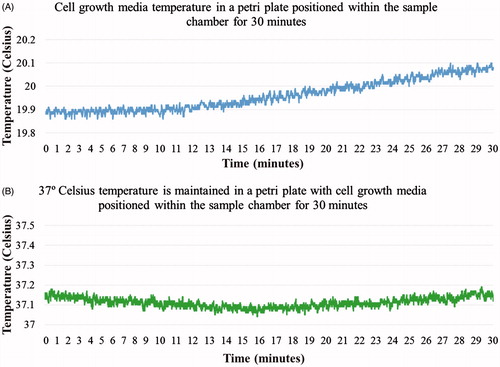
Figure 5. (A–D) Phase contrast and fluorescent images of SH-SY5Y cells. (A) No AMF, (B) AMF for 30 min, (C) 2 mg/mL MNP + No AMF, (D) 2 mg/mL MNP + AMF for 30 min. (I) Phase contrast, (II) Hoechst stain, (III) propidium iodide. (E) Bar chart showing the percentage of cytotoxicity in SH-SY5Y cells under different conditions (No AMF, AMF for 30 min, 2 mg/mL MNP + No AMF, 2 mg/mL MNP + AMF for 30 min). Data shown are the mean ± SD of N = 3.
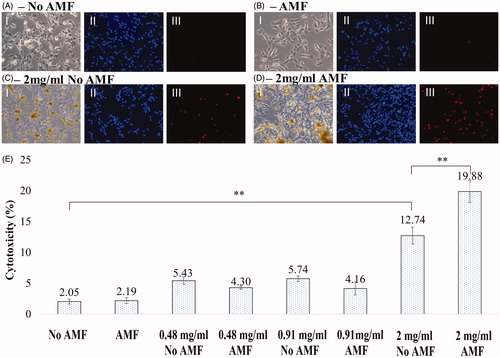
Figure 6. AMF-mediated nanoactuation set-up (planar coil) mounted onto different types of microscope. (A) Leica inverted florescence microscope, (B) Brunei inverted optical microscope, and (C) Zeiss upright microscope.
Figure 7. Magnetic flux density magneto static simulation for (A) six-turn planar zx plane, and (B) eight-turn planar zx plane coil at 57 A current rms. (C) Magnetic flux density z axis line diagram for six-turn planar zx plane, eight-turn planar zx plane at 36 A and 57 A current rms.
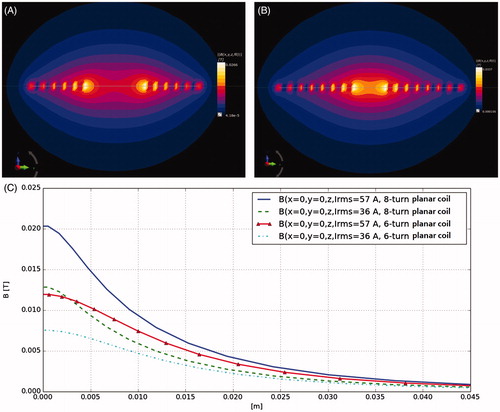
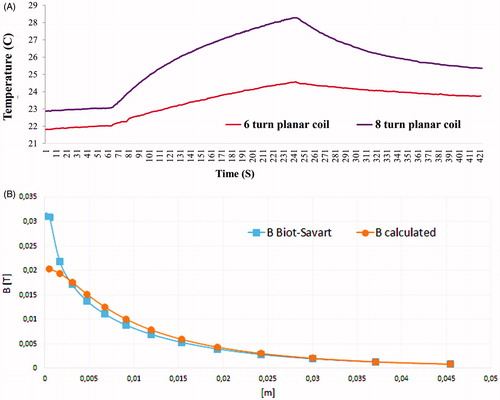
Figure 8. (A) Change of temperature over time curve for 8-turn planar coil set-up (218.7 kHz; at 57 A current rms) when compared to 6-turn planar coil set-up (223.1 kHz; at 57 A current rms). AMF exposure time was 180 s. (B) Magnetic flux density in z axis for 8-turn planar coil at 57 A current rms. Numerical model calculations compared to analytical method (derived from Biot-Savart law) calculations.
The results of the calorimetric experiment conducted to ensure that the system could enable heat dissipation within MNPs when exposed to AMF are shown in . illustrates the increase in temperature over time when subject to AMF from the planar coil set-up together with corresponding SAR values for the fields and frequencies tested. We were able to demonstrate that there was no non-specific heating when we exposed ddH2O controls to corresponding AMFs () and a corresponding increase in SAR as a function of the AMF frequency at constant field strength (5 mT) in Hypermag particles ().
Cell growth media in a Petri plate was maintained at 37 °C physiological temperature when positioned within the cell culture chamber as shown in . An AMF exposure microscopy experiment was successfully performed on SH-SY5Y neuroblastoma cells incubated with different concentrations of Hypermag nanoparticles using the planar coil set-up. Cytotoxicity of the SH-SY5Y cells for different conditions is shown in . From the results it is evident that AMF exposure did lower viability significantly, i.e. 2 mg/mL of Hypermag only compared to 2 mg/mL of Hypermag + AMF exposure for 30 min (**p < 0.01). However, the DMSA-stabilised aqueous suspension of 15.2 nm sized 10 mg/mL MNPs were toxic to the cells on their own with increase in concentration, i.e. 2 mg/mL of Hypermag only compared to No AMF control (**p < 0.01).
shows that the system could be mounted onto an inverted fluorescence microscope, an upright florescence microscope and an optical microscope, of various manufacturers, proving the compatibility of the design. We further enhanced the efficiency of the planar coil system by decreasing the coil radius to 6 mm and increasing the number of turns to eight as shown in . The contour plot and the line diagram () shows that field intensity and homogeneity experienced by the cells within the chamber is better in the eight-turn planar coil set-up. Frequencies achievable are provided in . About a twofold increase in results was obtained using the eight-turn planar coil set-up as shown in . Flux density results obtained from analytical Equation (Equation1(1) ) were compared with values obtained from the numerical investigation as shown in .
Table 2. Frequencies achievable with the nanomagnetic AMF actuation set-up for time lapse imaging design; 4-turn solenoid coil (L = 1.6454 µH), 6 turn planar coil (L = 2.5434 µH) and 8 turn planar coil (L = 2.6454 µH).
The flux density was 21 mT at the centre and 9.5 mT at 10 mm along the z axis when the current was 57.3 IRMS, and 13 mT at the centre of the coil decreasing to 6 mT at 10 mm along the z axis when the current was 36.1 IRMS based on the flux density simulations. Tracks were added to hold the coil connections together for cancelling out any losses as shown in . Our results indicated that the microscope hardware used in this arrangement will experience negligible fields in the x or y directional axes and that in the z direction the optics will experience 2 to 4 mT of stray field at 21 mm (+2.1 cm) away from the coil centre (the closest setting for the optics used), and 1 to 2 mT of stray field at 31 mm (+3.1 cm) away from the coil centre (the farthest setting for the optics used). This stray field had very low or no effect on the optics due to their geometry and the very low area on the upper surface that is being exposed to AMF. This stray field was completely eliminated by placing a RF shield gasket made of polymer on top of the optics with a lens aperture.
Discussion and conclusions
The design of the system described here should enable systematic studies of cell-level effects of MFH as well as RF activation of MNP-mediated cell signalling at physiologically relevant fields and frequencies. We have demonstrated one such experiment in this study, i.e. enhanced cytotoxicity in SH-SY5Y cells mediated by MNPs with AMF exposure. The cytotoxicity observed in the MNPs-only control can be related to the large volume of MNPs internalised through endocytic pathways per cell [Citation9] or due to incompatibility of MNPs with the semi adherent SH-SY5Y cells. Even though the MNPs provide a high SAR value, most of the heating is due to the stirring effect for the 15.2 nm MNPs (Hypermag) used in this study.
It has been demonstrated earlier that once Hypermag is embedded within wax there was a significant drop in the SAR value, possibly indicating a Brownian relaxation mechanism at these frequencies [Citation10]. Moreover, a recent study states that when MNPs attach to the cell membrane or when they are internalised by cells, the SAR value drops [Citation9] and an increased amount of iron content must to be internalised by numerous cells in order to achieve a therapeutic temperature rise [Citation11]. Hence, the amount of heat dissipated by the internalised MNPs and MNPs attached to the surface would be low during the brief AMF exposure period. This may be the reason for the low levels of cytotoxicity observed in our experiments when comparing 2 mg/ml MNPs only and 2 mg/ml MNPs with AMF conditions in SH-SY5Y cells.
There are numerous parameters to be analysed in vitro before magnetic fluid hyperthermia translates into a successful clinical application, such as MNP specificity, MNP cytotoxicity, MNP localisation, heat dissipation mechanisms of MNPs within the cells and tissue, repeated AMF exposure and its side effects on healthy cells, cellular microenvironment changes due to surface functionalised MNPs, and AMF, ion channel influx/efflux, MNP–AMF mediated heat shock protein release, long-/short-term exposure of RF electric field effects on cells. Perhaps most importantly, phenotypic and genotypic changes on the cells/tissue slices could be studied real-time in individual cells using this RF-AMF micro-stage design.
In addition to nanomagnetic actuation and magnetic fluid hyperthermia microscopy studies, this device could also be used for research on AMF-triggered drug release using thermo-labile MNPs and conjugated drugs [Citation12,Citation13], triggered thermal activation of shape memory polymers embedded with MNPs for biomedical applications [Citation14,Citation15], and AMF-mediated biofilm inactivation using iron oxide nanoparticles [Citation16].
Likewise, heat dissipation by MNPs will also depend on the incident amplitude, intensity and frequency parameters. Herein we have used an eight-turn planar coil leading current Irms = 57 A, as shown in . Note that in the analytical formulae (Equations Equation1(1) and Equation2
(2) ) it is assumed that the wire diameter is infinitely small, whereas in the numerical model it is not, i.e. in our case the wire diameter was 1.22 mm. The working distance of the objective used in this study was 10 mm. The positioning of the cell culture Petri plate depends extensively on that distance, which was the primary factor for choosing the planar coil set-up. However, the efficiency of both systems, especially the solenoid coil system, can be drastically enhanced if longer working distance microscope optics are used. Imaging is possible using objectives available for 30 mm or more working distance by elevating the position of the Petri dish to the centre of the coil. Using such a modified arrangement could expose the cells to a stronger homogenous field of increased intensity and eliminate any stray field effects on the z axis. This will be the subject of further experiments in our future research.
Few magnetic field exposure systems have been developed for magnetic actuation research [Citation17,Citation18,Citation19]. However, we took into account their drawbacks and fabricated this design with the intention of operating at radiofrequencies, enabling increased flux density and flux intensity when exposing cells, non-contact AMF exposure on cells to avoid contamination, a fixed sample chamber to enable repeatability of AMF–cell culture experiments, compatibility for microscopy, time lapse imaging in a 37 °C environment with bio gas ability, and fixed coil geometry to reproduce measurements.
The RF coil chamber described here provides a simple yet highly effective means for real-time observation and monitoring of cell death in vitro. Our experimental results indicate that cells can be maintained in a healthy state during microscopy observation and AMF exposure by providing control over physiological temperature and CO2 concentration. The operating parameters make this system well suited to MFH and RF actuation studies at the cellular level.
Acknowledgements
We would like to thank ISTM, Keele University for allowing us to test our setup compatibility with the microscopes available within their facility.
M.S. conceived, designed and engineered the set-up. M.S. and A.M. performed the evaluation experiments. M.S., A.M. and J.D. performed the data analysis. M.S. wrote the manuscript. G.P. and J.D. edited the manuscript.
Declaration of interest
The research was funded by nanoTherics Ltd. nanoTherics is involved with commercialisation of the coil system for real-time magnetic fluid hyperthermia microscopy studies. M.S. works as a research scientist in biomedical engineering for nanoTherics, and J.D. is a consultant for nanoTherics. A.M. and G.P. are academic researchers and are unrelated to nanoTherics Ltd. The authors alone are responsible for the content and writing of the paper.
References
- Creixell M, Bohorquez AC, Torres-Lugo M, Rinaldi C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011;5:7124–9
- Ivkov R. Magnetic nanoparticle hyperthermia: A new frontier in biomedicine? Int J Hyperthermia 2013;29:703–5
- Jordan A, Scholz R, Wust P, Fähling H, Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 1999;201:413–19
- Kozissnik B, Bohorquez AC, Dobson J, Rinaldi C. Magnetic fluid hyperthermia: Advances, challenges and opportunity. Int J Hyperthermia 2013;29:706–14
- Pankhurst QA, Connoly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2003;36:R167–81
- Pankhurst QA, Thanh NTK, Jones SK, Dobson J. Progress in applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 2009;42:224001
- Zahn M. Electromagnetic Field Theory: A Problem Solving Approach. Malabar, FL: Krieger, 2003
- Bordelon DE, Cornejo C, Grüttner C, Westphal F, DeWeese TL, Ivkov R. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterised with wide ranging and high amplitude alternating magnetic fields. J Appl Phys 2011;109:124904
- Corato RD, Espinosa A, Lartigue L, Tharaud M, Chat S, Pellegrino T, et al. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 2014;35:6400–11
- Vallejo-Fernandez G, Whear O, Roca AG, Hussain S, Timmis J, Patel V, et al. Mechanisms of hyperthermia in magnetic nanoparticles. J Phys D Appl Phys 2013;46:31:312001
- Hedayati M, Thomas O, Abubaker-Sharif B, Zhou H, Cornejo C, Zhang Y, et al. The effect of cell cluster size on intracellular nanoparticle-mediated hyperthermia: Is it possible to treat microscopic tumors? Nanomedicine 2013;8:29–41
- Riedinger A, Guardia P, Curcio A, Garcia MA, Cingolani R, Manna L, Pellegrino T. Subnanometer local temperature probing and remotely controlled drug release based on azo-functionalized iron oxide nanoparticles. Nano Lett 2013;13:2399–406
- Savva I, Odysseos AD, Evaggelou L, Marinica O, Vasile E, Vekas L, et al. Fabrication, characterization and evaluation in drug release properties of magnetoactive poly (ethylene oxide)-poly(l-lactide) electrospun membranes. Biomacromolecules 2013;14:4436–46
- Buckley PR, McKinley GH, Wilson TS, Small W, Benett WJ, Bearinger JP, et al. Inductively heated shape memory polymer for the magnetic actuation of medical devices. IEEE Trans Biomed Eng 2006;53:2075–83
- Mohr R, Kratz K, Weigel T, Lucka-Gabor M, Moneke M, Lendlein A. Initiation of shape–memory effect by inductive heating of magnetic nanoparticles in thermoplastic polymers. Proc Natl Acad Sci USA 2006;103:3540–5
- Park H, Park HJ, Kim JA, Lee SH, Kim JH, Yoon J, Park TH. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J Microbiol Methods 2011;84:41–5
- Connord V, Clerc P, Hallali N, El Hajj Diab D, Fourmy D, Gigoux V, et al. Real-time analysis of magnetic hyperthermia experiments on living cells under a confocal microscope. Small 2015;11:2437–45
- Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat Nanotechnol 2010;5:602–6
- Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, et al. Nanomagnetic actuation of receptor-mediated signal transduction. Nat Nanotechnol 2008;3:1:36–40
