Abstract
Mild hyperthermia (HT) (41.5 °C for 30–60 min) has been shown in various cell culture systems, preclinical and clinical models to be a very potent radiosensitiser. Recent research suggests that local HT application in combination with standard tumour therapies such as radiotherapy (RT) and/or chemotherapy may not only improve local tumour control but also lead to systemic and immune mediated anti-tumour responses. Melanoma has been proven to be rather radioresistant and mostly only the addition of immunotherapy is capable of inducing beneficial anti-melanoma responses. This work therefore focuses on whether HT increases the immunogenic potential of B16-F10 mouse melanoma cells in combination with RT. The in vitro experiments revealed that combination of RT with HT resulted in an increased percentage of apoptotic and necrotic melanoma cells and an increased release of the danger signal heat shock protein 70 (Hsp70) and high mobility group box protein 1 (HMGB1). HT alone was also capable of inducing this release. We set up local irradiation and heating procedures of B16-F10 tumour-bearing C57/BL6 mice and revealed that the tumour growth of tumours treated with RT plus HT was significantly retarded compared to tumours treated only with RT. This combined treatment generated a beneficial tumour microenvironment by enhancing the infiltration of CD11c + /MHCII + /CD86+ dendritic cells, CD8+ T cells, and NK cells, and decreasing that of regulatory T cells and myeloid-derived suppressor cells. We conclude that HT in combination with RT has an immune-stimulating potential that might result in anti-tumour immunity.
Introduction
Anti-cancer therapies aim to stop the proliferation of tumour cells, ultimately kill them and ideally induce systemic anti-tumour immunity [Citation1]. The latter is beneficial for destroying the primary tumour, but also for preventing metastases and recurrences. Cancer cells can be rendered visible to the immune system by standard therapies such as radiotherapy (RT) or chemotherapy (CT), especially in combination with further immune stimulation [Citation2]. One way to activate the immune system is the application of hyperthermia (HT) [Citation3,Citation4]. Two prominent forms of HT, namely whole body HT and local HT, are applied [Citation5–7]. The local deep regional HT is used as an adjuvant for treatment of cancer with RT, CT or radiochemotherapy (RCT). It inhibits DNA repair of irradiation-induced double strand breaks [Citation8], sensitises the tumour cells for irradiation when it is delivered before RT or aggravates the irradiation-induced cellular stress when applied after RT, and enhances the effects of certain chemotherapeutic agents, to mention just some of the local modes of action of HT [Citation9,Citation10].
Besides its main local anti-tumour effects, HT also fosters systemic anti-tumour immune responses. It enhances the expression of heat shock proteins (HSPs) that are relevant, when released, for the induction of anti-tumour immunity [Citation11–13]. Inside the cells, HSPs protect the cells from cellular stress. They act as chaperones and thereby stabilise proteins, or can ubiquitinate damaged proteins, ultimately leading to their degradation in the proteasome. Of note is that outside the cell, HSPs can efficiently activate the immune system. Many HSPs, such as Hsp70, bind tumour antigens. When Hsp70 is released it delivers the bound antigen to antigen presenting cells (APCs). The latter internalise the HSPs and thereby also the antigens by receptor-mediated endocytosis. Finally, the tumour antigens are cross-presented via MHCI molecules, especially by dendritic cells (DCs), and stimulate the CD8+ T-cell response in this way [Citation14]. Another immune-stimulatory effect of HSPs is that they can induce the secretion of pro-inflammatory cytokines by APCs such as DCs [Citation15]. In summary, extracellular HSPs act as danger signals (DAMPs), resulting in maturation and activation of APCs. The secretion of Hsp70 by tumour cells is significantly increased after exposure to a combination of radiotherapy with hyperthermia [Citation16], while RT alone specifically increases the surface exposure of Hsp70 on tumour cells [Citation17]. Here, Hsp70, even in the absence of immunogenic peptides, is a main target structure for cells of the innate immune system, namely for activated natural killer (NK) cells [Citation18,Citation19]. Furthermore, HSPs might co-operate with the mismatch repair systems [Citation20]. This again highlights that local and systemic anti-tumour effects are inducible by HSPs.
There are initial indications that HT impacts not only on the release of Hsp70 [Citation21] but also on that of the DAMP high mobility group box 1 (HMGB1). Under physiological conditions HMGB1 is ubiquitously expressed in the nucleus of mammalian cells and is highly conserved between different species. It acts as a non-histone chromatin-associated protein, binding to DNA and facilitating the binding of transcription factors [Citation22]. Another function is its role in the recognition of DNA damage in the process of mismatch repair [Citation23]. When danger occurs, proteins located inside the cells may spill out and thereby become immunogenic [Citation24]. Since necrotic cells lose their membrane integrity, HMGB1 is passively released [Citation25,Citation26], but inflammatory cells also secrete HMGB1 actively [Citation27]. Standard therapies such as RT or CT can induce the release or secretion of HMGB1 [Citation28] which in turn activates DCs; the latter subsequently prime naïve T cells [Citation29]. HMGB1, especially, binds to the receptor for advanced glycation end products (RAGE) and to Toll-like receptors (TLR) 4 and 2 [Citation30]. It has already been suggested, more than 40 years ago, that necrosis induction by combination of RT with HT might be the main trigger for induction of anti-tumour immunity [Citation31].
Since melanoma is the tumour entity with the highest prevalence of somatic mutations across human cancer types [Citation32] it can be specifically targeted by immune cells after rendering the tumour immunogenic through therapy. Melanoma cells are also sensitive to heat [Citation33]. A randomised trial comparing RT with or without HT showed that the addition of hyperthermia to RT increased the complete response rate from 35–62% and two-year local control rates from 28–46% [Citation34]. We showed recently that modulation of therapy-induced tumour cell death with the pan-caspase inhibitor zVAD-fmk induces anti-melanoma immunity in a HMGB1-, nucleotide- and T-cell-dependent manner [Citation35]. We are now interested in whether HT is already capable of rendering irradiated melanoma cells more immunogenic.
Material and methods
Cell culture
The mouse melanoma cell line B16-F10 (ATCC, Manassas, VA, USA) was derived from C57/BL6 mice and cultured in RPMI 1640 medium with stable glutamine (Biochrom, Berlin, Germany) supplemented with 10% heat inactivated fetal bovine serum (Biochrom), 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Carlsbad, CA). The cells tested negative for mycoplasma contamination by PCR detection kit were kept in 5% CO2 atmosphere at 37 °C and 95% relative humidity. The cells were used when they reached 90% confluence.
Treatment of B16-F10 melanoma cells
The tumour cells were irradiated with an X-ray generator (GE Inspection Technologies, Hürth, Germany, 120 kV, 22.7 mA, 0.5 min) with the clinically relevant single dose of 2 Gy for RT of tumours. For HT, the B16-F10 melanoma cells were treated in a homemade device placed in a cell incubator as described previously [Citation16]. The cells remained at 41.5 °C for 60 min. The variations of the temperature during the treatment were less than 0.2 °C. For combined applications the tumour cells were stored at 37 °C for 4 h between RT and HT treatment, as this is the common maximum interval in clinical application.
Cell death determination
Melanoma cell death forms were determined by annexin A5 (Anx5)-FITC and propidium iodide (PI) staining. For analysis of cell death, 1 × 105 cells were transferred in 400 μL of Ringer’s solution (Braun, Melsungen, Germany) containing 0.2 µg AnxA5-FITC and 0.4 µg PI. After 30 min of incubation at 4 °C, the samples were analysed by two-colour flow cytometry. AnxA5 protein was expressed and produced in 293 human embryonic kidney cells (FreeStyle™ 293 Expression System, Life Technologies, Regensburg, Germany) and purified. Labelling with fluorescein isothiocyanate (FITC) was performed with the FluoroTag™ FITC Conjugation Kit (Sigma Aldrich, St Louis, MO) according to the manufacturer’s instructions. AnxA5−PI− double negative cells were defined as viable, AnxA5+PI− as apoptotic cells, and AnxA5+PI+ as necrotic cells [Citation36] ().
Detection of danger signals
To analyse extracellular danger signals, supernatants (SN) of B16-F10 cells were collected 24 h after the respective treatment. SN were then analysed by ELISA for HMGB1 (Shino-Test Corporation, Tokyo, Japan) and Hsp70 (R&D Systems, Minneapolis, MN). The latter was also applied for analysis of Hsp70 in mouse serum. ELISAs were performed according to the manufacturer’s instructions.
Induction of B16-F10 melanomas in C57/BL6 mice
For analysis of in vivo tumour growth of syngeneic tumours, eight-week-old female C57/BL6 mice were used. 106 B16-F10 cells in 200 µL Ringer’s solution were injected into the right shaved flank of the mice on day zero. The tumour volumes were monitored over the following days. To do so, width and length were measured using a digital caliper and tumour volume was calculated according to the following formula: volume (mm3) = 0.5 × width2 (mm2) × length (mm) [Citation37]. The mice were bred in a sterile atmosphere at the animal facility of Friedrich-Alexander-Universität Erlangen-Nürnberg (Franz Penzoldt Center). The animal procedures were approved by the government of Middle Franconia and were conducted in accordance with the guidelines of the Federation of European Laboratory Animal Science Associations (FELASA).
Treatment of B16-F10 melanomas with ionising radiation and hyperthermia
On days 8, 9 and 10 the irradiation was performed. The tumours were locally irradiated with a clinically relevant single dose of 2 Gy using a PRIMART linear accelerator (6MV, Siemens, Munich, Germany). Local hyperthermia was delivered on days 8 and 10, in each case 4 h after RT. To do so the mice were anaesthetised and the tumours were heated locally with microwaves to 41.5 °C for 30 min using a BSD50 Hyperthermia System (BSD Medical, Salt Lake City, UT) (see also , establishment of local treatment procedures).
Analysis of immune cell infiltration into the tumour
For analysis of immune cell infiltration, the tumours were prepared with a tumour dissociation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Afterwards the tumour cells were centrifuged with Easycoll separating solution (Biochrom) to discard dead cells. The cell suspension was then stained for 30 min at 4 °C with the following fluorescence-labelled antibodies: CD4-PCC5.5 (BD Pharmingen, New York), CD8-PE (BD Pharmingen), CD3-V450 (BD Pharmingen), CD11c-PE (BD Pharmingen), MHCII-eF450 (eBioscience, Frankfurt, Germany), CD86-AF700 (BD Pharmingen), CD11b-PE (BD Pharmingen), Ly6G-BDH450 (BD Pharmingen), CD45.2-PCC5.5 (eBioscience), CD45R-PCC5.5 (BD Pharmingen), NK1.1-APC (eBioscience). Determination of Tregs was performed with the FoxP3 Staining Buffer Set and the antibodies CD4-Vioblue, CD25-AF488 and FoxP3-APC (all from Miltenyi Biotec). Multicolour flow cytometry was performed with a Gallios® Flow Cytometer and the data obtained were analysed using Kaluza analysis software v. 1.3. (both Beckman Coulter, Krefeld, Germany).
Statistical analysis
GraphPad Prism (version 5.04, La Jolla, CA) was applied for statistical analysis with the Mann-Whitney U test. Results were considered statistically significant for p < 0.05 (*) and highly significant for p < 0.01 (**).
Results
HT in combination with RT generates a mixture of apoptotic and necrotic melanoma cells
The response of melanoma cells to classical RT with ionising radiation (X-ray) is often very poor [Citation38]. However, there are indications that HT is capable of increasing the efficacy of RT also in this tumour entity [Citation39]. Therefore, we tested whether HT alone or particularly in combination with RT induces apoptosis or necrosis in B16-F10 melanoma cells, since a mixture of these classical tumour cell death forms is especially immunogenic [Citation40].
A single irradiation of the melanoma cells with 2 Gy of X-ray did not significantly alter the cell death rates, neither of apoptosis nor of necrosis one day after treatment. Heating the tumour cells for 1 h to 41.5 °C resulted in increased apoptotic cell death, but not of necrotic cell death. However, apoptotic and necrotic melanoma cells were significantly increased when combining RT with HT ().
Figure 1. Cell death and release of danger signals of melanoma cells after irradiation and hyperthermia. The cell death forms of B16-F10 melanoma cells were analysed with two-colour flow cytometry after staining of the cell suspension with AnxA5-FITC and PI, 24 h after treatment with RT (2 Gy), HT (41.5 °C for 1 h) or a combination of both. The percentage of apoptotic cells (positive for AnxA5 binding and negative for PI staining) is displayed in A, and that of necrotic cells (positive for both, AnxA5 binding and PI staining) in B. The fold increase of HMGB1 (C) and Hsp70 (D) compared to mock controls in the supernatants of the tumour cells was determined with the ELISA technique after the respective treatments. Representative data of one out of three experiments, each performed in triplicate (A, B) or duplicate (C, D), are presented as mean ± SD. *p < 0.05; **p < 0.01.
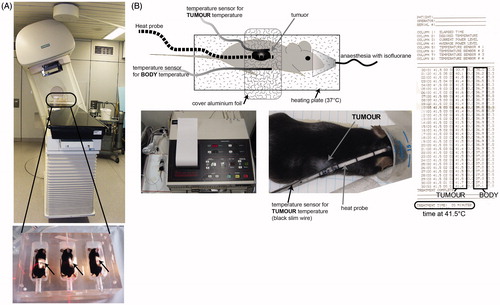
Figure 2. Establishment of local treatment procedures for radiotherapy and hyperthermia of tumour-bearing mice. To locally irradiate the B16-F10 tumour -bearing C57/BL6 mice, a Plexiglas® box was manufactured which allows the irradiation of three mice at once (A). The mice were anaesthetised before being placed in the box. For the irradiation procedure, the mice were kept under isoflurane anaesthesia to avoid them moving. The planning of the irradiation was conducted using a computer tomography image of the irradiation box and tumour-bearing mice (not displayed) with Philips pinnacle software to obtain an optimal target volume. Afterwards the dosimetry of the irradiation was performed manually with a calibrated ionisation chamber. To further protect normal tissue the gantry of the 6 MV linear accelerator was rotated to 340°. The light field control of the irradiation field for the tumour-bearing mice (arrows) is displayed in the lower image of A. For local hyperthermia (B), the mice were also anaesthetised and the tumours were heated with microwave catheters to 41.5 °C for 30 min using the BSD50 hyperthermia system. On the other side of the tumour temperature was controlled with a sensor. To prevent cooling of the body, the mice were placed on a plate which was heated with warm water at 37 °C (heating plate). The body temperature of the mice was controlled with another temperature sensor. To improve the heating of the tumours, the mice were covered with aluminium foil. Both the local temperature in the tumour and the body temperature were continuously controlled.
HT alone and in combination with RT enhances the release of the danger signals HMGB1 and Hsp70
We were then interested whether the immunogenic potential of the B16-F10 cells is influenced by HT and/or RT. To gain initial indications, we analysed the release of the danger signals HMGB1 and Hsp70 by the tumour cells. Both HT alone and HT in combination with RT enhanced the release of both danger signals significantly, while RT alone did not impact on it ().
Establishment of local treatment procedures of RT and HT closely resembling the clinical situation
Pre-clinical in vivo model systems closely resembling the clinical situation are urgently needed to analyse the immunogenic potential of local applications [Citation41]. Therefore, we established a local irradiation and also a local hyperthermia treatment for B16-F10 tumour-bearing C57/BL6 mice. The local irradiation was delivered with a linear accelerator (PRIMART, 6 MV, Siemens). The tumours were locally irradiated (arrows in ) with a clinically relevant single dose of 2 Gy. Treatment planning was conducted using a computer tomography image of the Plexiglas irradiation box and tumour-bearing mice with Philips pinnacle software (Best, Netherlands). To protect normal tissue the gantry of the linear accelerator was rotated to 340° ().
For local hyperthermia, the BSD50 apparatus was used. It is based on transurethral microwave technology (TUMT) and was originally used for the treatment of prostate cancer. We adapted it for local treatment of ectopic mouse tumours () and the B16-F10 tumours were heated with microwave catheters that lay directly on the tumour. On the other side of the tumour a sensor controlled the temperature of the tumour throughout the treatment period. To prevent hypothermia of the body, the mice were laid on a heating plate which was heated with 37 °C warm water during the local HT treatment. The body temperature of the mice was controlled with another temperature sensor. To improve the heating of the tumours the mice were covered with aluminium foil. Both the local temperature and the body temperature were controlled throughout the treatment period ().
Fractionated RT alone does not impact on tumour growth, but combination with HT retards tumour growth significantly
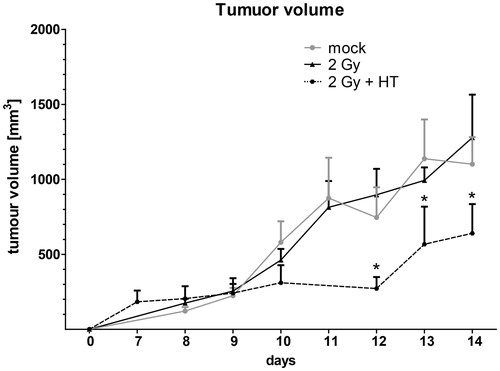
Treatment of tumour-bearing mice with fractionated irradiation with 3 × 2 Gy (RT) did not affect the tumour growth significantly; the tumour growth was comparable to that of untreated animals. However, combination of hyperthermia with fractionated RT reduced the tumour growth significantly ().
Figure 3. Impact of fractionated radiotherapy and hyperthermia on tumour volume of B16-F10 tumours in C57BL6 mice. The growth of syngeneic B16-F10 tumours in wild-type C57/BL6 mice is displayed. The tumours were locally irradiated on days 8, 9 and 10 with a clinically relevant single dose of 2 Gy using a linear accelerator. Hyperthermia (HT) was performed 4 h after irradiation on days 8 and 10. For this, the mice were heated locally under temperature control to 41.5 °C for 30 min. An electronic caliper was used to monitor the tumour volume. Joint data of three independent experiments, each with two mice per group, are presented as mean ± SD. *p < 0.05 related to mock-treated tumours.
Combinatory treatment of fractionated RT with HT generates a beneficial immune cell infiltrate in the tumours
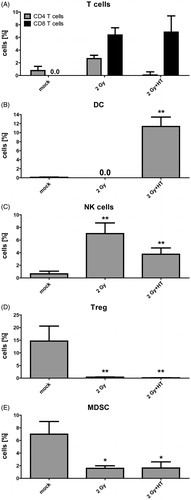
In tumours of untreated mice low amounts of CD4+ T cells and no CD8+ T cells were observed. Neither fractionated RT alone nor fractionated RT in combination with HT altered the infiltration of CD4+ T cells into the tumour significantly. In contrast, both fractionated RT alone and fractionated RT in combination with HT resulted in infiltration of CD8+ T cells (). Of note is that DCs were only present in the tumour after combination of fractionated RT with HT (). RT also induced a significantly increased percentage of NK cells in the tumour. However, combination of RT with HT only resulted in a slight but significant increase of NK cells (). Regarding immunosuppressive cells such as regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), both fractionated RT alone and fractionated RT in combination with HT reduced their amount in the tumour to less than 1% (Treg) or less than 3% (MDSC), respectively ().
Figure 4. Impact of fractionated radiotherapy and hyperthermia on immune cell infiltration into B16-F10 tumours. (A) The infiltration of immune cells (CD4+ or CD8+ T cells), (B) dendritic cells (DC), (C) natural killer (NK) cells, (D) regulatory T cells (Treg), and (E) myeloid-derived suppressor cells (MDSC), into B16-F10 tumours on C57/BL6 mice was analysed by multicolour flow cytometry 24 h after treatment with fractionated radiotherapy (RT) with a single dose of 2 Gy and fractionated RT plus HT. Joint data of three experiments, each performed in duplicates, are presented as mean ± SD. *p < 0.05; **p < 0.01 related to mock treated tumours.
Discussion
RT is one of the most frequently used standard treatments for cancers and 60% of all cancer patients receive it nowadays [Citation42]. Unfortunately, melanoma is a very radioresistant and chemoresistant tumour entity [Citation38,Citation43]. Therefore, primary melanomas are mostly treated with surgical resection which, however, does not strongly impact on the immune system. Although surgery is a very good option to fight the primary tumour, it cannot prevent metastases and recurrences. For this reason an additional immune activation is important [Citation44]. Immune checkpoint inhibitors such as anti-CTLA-4 and anti-PD1 antibodies have already made their way into clinics and improve the survival of melanoma patients significantly [Citation45]. Nevertheless, strong autoimmune reactions might also be induced and the most beneficial chronology and combination with RT must first be identified [Citation46,Citation47]. Therefore, there is also a strong need to identify immune modulators with fewer side effects. One promising strategy is the combination of standard therapies, namely RT and/or CT, with HT [Citation7]. One mechanism by which HT contributes to activation of the immune system is the induction of immunogenic tumour cell death forms when combined with RT. These are characterised by the release of danger signals [Citation16,Citation48,Citation49]. Besides necrosis, HT is capable of inducing caspase-dependent and independent apoptosis [Citation50,Citation51]. A mixture of both cell death forms, namely apoptosis and necrosis, is especially immunogenic [Citation40,Citation52]. We found that only RT in combination with HT increased the amount of both apoptotic and necrotic melanoma cells (). Necrosis induction of melanoma cells and release of HMGB1 has also been observed when combining RT with the pan-caspase inhibitor zVAD-fmk [Citation35]. The danger signals were also released in higher amounts when B16-F10 melanoma cells were treated with HT (). Of note is that no correlation with necrosis induction exists in this case. This suggests that HT-induced melanoma apoptosis is also immunogenic, and apoptotic blebs might be carriers of the danger signals as already described for exosomes [Citation53–55]. Yang et al. recently demon-strated that heat stress increases the quantity of doxorubicin-containing exosomes from tumour cells and thereby enhances anti-tumour effects [Citation56]. Future experiments should therefore also focus on measurement of liposomal bound Hsp70. This has become possible since the development of a lipHsp70 ELISA [Citation57]. ELISAs for detection of HMGB1 in exosomes are also currently under development.
Furthermore, we succeeded in setting up local RT and HT tumour treatment procedures () that are needed for preclinical testing of the combination of RT with additional immune therapy [Citation58]. In our in vivo setting we demonstrated that only fractionated RT in combination with HT significantly retarded tumour growth (). Although the in vitro experiments revealed that HT treatment alone results in increased extracellular concentrations of the immune activating danger signals Hsp70 and HMGB1 () we did not include this in our in vivo analyses. This was due to the demands of the party responsible for animal procedures. Since local HT treatment is only applied and proven to be beneficial in combination with CT and/or RT in clinics [Citation59], only the combined treatment of RT with HT was approved for the mouse experiments. Hyperthermia-induced tumour oxygenation might further contribute to enhanced efficacy of RT [Citation60]. However, since in our model system HT was delivered 4 h after RT to focus on aggravation of the irradiation-induced cellular stress, this should play a subordinated role. Improved tumour oxygenation by HT is generally transient and often no longer evident as early as 1 h after heating [Citation61].
HT in combination with RT not only directly impacted on the tumour by reducing its size (local tumour control), but also, in particular, enhanced the infiltration of DCs into the tumour when combined with fractionated RT (). The CD11c + /MHCII + /CD86 + DC population was analysed to gain initial indications of the infiltration of APCs into the tumour. To further define the immunogenicity of the infiltrating DCs additional functional ex vivo experiments will be performed in the future. In general, activated APCs are essential for triggering innate and adaptive anti-tumour immune responses including those mediated by HSPs [Citation15,Citation62]. Since the infiltration of cytotoxic CD8+ T cells (CTLs) that exert tumour cell killing after stimulation by danger signal-activated DCs [Citation63] was induced by RT alone and even slightly more when combined with HT (), we conclude that activated DCs rather than tolerogenic ones had infiltrated into the tumour. RT also induced a significantly increased percentage of NK cells in the tumour. However, combination of RT with HT only resulted in a slight increase of NK cells in the tumour (). Furthermore, a slight, but not significant increase of serum Hsp70 was observed when combining RT with HT compared to RT alone (supplementary Figure 1). This suggests that the reduced tumour growth induced by combination of RT with HT is at least in part immune-mediated. However, future experiments with immune deficient mice are needed to provide final proof of this. Nevertheless, we have recently demonstrated that a triple combination of RT with HT and the chemotherapeutic agent dacarbazine results in significantly reduced growth in both wild type (WT) and RAG KO mice [Citation35]. However, in the latter the tumour growth retardation was less pronounced than in WT mice. This again suggests that both immune dependent and immune independent mechanisms contribute to therapy-induced tumour growth retardation. Additionally, NK cells might also be involved in anti-tumour reactivity. This needs to be examined in future experiments with NK cell depleting antibodies.
Future work should also focus on measurement of liposomal bound Hsp70 that might especially be released after radioimmunotherapy [Citation57]. A dynamic immune modulation by RT plus HT was recently described by Datta et al. for liposarcoma [Citation4]. However, the melanoma microenvironment particularly has been characterised by accumulation of immunosuppressive regulatory leucocytes, in particular MDSC [Citation64]. We also observed in our B16-F10/C57/BL6 model that MDSC and Treg are present in the tumour. Unlike CD8+ T cells and DCs, the percentage of these immunosuppressive cells was decreased in the tumour after fractionated RT alone and in combination with HT ().
This suggests that RT in combination with HT induces a tumour microenvironment which is immunogenic and characterised by apoptotic and necrotic tumour cells, presence of the danger signals HMGB1 and HSP70, enhanced infiltration of CD8+ T cells, DCs, and NK cells, and concomitantly decreased infiltration of Treg and MDSC. HT combined with RT can therefore be regarded as a potential in situ tumour vaccinator [Citation62]. Future research should focus on the best combination of HT with distinct fractionation schemes of RT and analyse in depth the related immune mechanisms. Combinations of RT with immune stimulations will increasingly make their way into clinics, not only for treatment of melanomas, as recently demonstrated by Golden et al. [Citation65]. Radiation in combination with immune modulators is capable of activating non-redundant immune mechanisms in cancer, and such next-generation combinations are worth following up [Citation58,Citation66]. HT should always be kept in mind as an immune stimulator and considered as an addition to RT and/or CT for the induction of not only improved local tumour control, but also anti-tumour immunity [Citation13,Citation67].
Supplementary materials available online
Acknowledgements
This work was supported by research training group GRK 1660 of the German Research Foundation (DFG) and by the German Federal Ministry of Education and Research (BMBF, m4 Cluster, 16EX1021R). We thank dR. Sennewald Medizintechnik GmbH for providing us with the BSD50 apparatus for local hyperthermia treatment of tumour-bearing mice and especially Günter Futschik for technical advice.
Declaration of interest
The authors report no other conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Scheithauer H, Belka C, Lauber K, Gaipl US. Immunological aspects of radiotherapy. Radiat Oncol 2014;9:185
- Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014;4:325
- Mace TA, Zhong L, Kokolus KM, Repasky EA. Effector CD8+ T cell IFN-gamma production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 2012;28:9–18
- Datta NR, Grobholz R, Puric E, Bode-Lesniewska B, Lomax N, Khan S, et al. Enhanced tumour regression in a patient of liposarcoma treated with radiotherapy and hyperthermia: Hint for dynamic immunomodulation by hyperthermia. Int J Hyperthermia 2015;31:574–7
- Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res 2013;1:210–16
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–39
- Krawczyk PM, Eppink B, Essers J, Stap J, Rodermond H, Odijk H, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci USA 2011;108:9851–6
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer 2008;44:2546–54
- van der Zee J. Heating the patient: A promising approach? Ann Oncol 2002;13:1173–84
- Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nat Med 1998;4:581–7
- Schilling D, Kuhnel A, Konrad S, Tetzlaff F, Bayer C, Yaglom J, et al. Sensitizing tumor cells to radiation by targeting the heat shock response. Cancer Lett 2015;360:294–301
- Lauber K, Brix N, Ernst A, Hennel R, Krombach J, Anders H, et al. Targeting the heat shock response in combination with radiotherapy: Sensitizing cancer cells to irradiation-induced cell death and heating up their immunogenicity. Cancer Lett 2015;368:209–29
- Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, et al. Cutting edge: Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol 1999;162:3757–60
- Torigoe T, Tamura Y, Sato N. Heat shock proteins and immunity: Application of hyperthermia for immunomodulation. Int J Hyperthermia 2009;25:610–16
- Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, et al. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol 2011;101:109–15
- Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, et al. A stress-inducible 72-kDa heat-shock protein (Hsp72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 1995;61:272–9
- Gehrmann M, Marienhagen J, Eichholtz-Wirth H, Fritz E, Ellwart J, Jaattela M, et al. Dual function of membrane-bound heat shock protein 70 (Hsp70), Bag-4, and Hsp40: Protection against radiation-induced effects and target structure for natural killer cells. Cell Death Differ 2005;12:38–51
- Multhoff G, Pockley AG, Schmid TE, Schilling D. The role of heat shock protein 70 (Hsp70) in radiation-induced immunomodulation. Cancer Lett 2015;368:179–84
- Sottile ML, Losinno AD, Fanelli MA, Cuello-Carrion FD, Montt-Guevara MM, Vargas-Roig LM, et al. Hyperthermia effects on Hsp27 and Hsp72 associations with mismatch repair (MMR) proteins and cisplatin toxicity in MMR-deficient/proficient colon cancer cell lines. Int J Hyperthermia 2015;31:464–75
- Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia 2013;29:399–408
- Tang D, Kang R, Zeh HJ III, Lotze MT. High-mobility group box 1 and cancer. Biochim Biophys Acta 2010;1799:131–40
- Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, et al. Masquerader: High mobility group box-1 and cancer. Clin Cancer Res 2007;13:2836–48
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol 2001;13:114–19
- Bustin M. At the crossroads of necrosis and apoptosis: Signaling to multiple cellular targets by HMGB1. Sci STKE 2002;151:pe39
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:1915
- Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002;3:995–1001
- Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, et al. High mobility group box I (HMGB1) release from tumor cells after treatment: Implications for development of targeted chemoimmunotherapy. J Immunother 2007;30:596–606
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13:1050–9
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004;279:7370–7
- Muckle DS, Dickson JA. Hyperthermia (42 °C) as an adjuvant to radiotherapy and chemotherapy in the treatment of the allogeneic VX2 carcinoma in the rabbit. Br J Cancer 1973;27:307–15
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 1996;12:3–20
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995;345:540–3
- Werthmoller N, Frey B, Wunderlich R, Fietkau R, Gaipl US. Modulation of radiochemoimmunotherapy-induced B16 melanoma cell death by the pan-caspase inhibitor zVAD-fmk induces anti-tumor immunity in a HMGB1-, nucleotide- and T-cell-dependent manner. Cell Death Dis 2015;6:e1761
- Gaipl US, Kuenkele S, Voll RE, Beyer TD, Kolowos W, Heyder P, et al. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ 2001;8:327–34
- Geran RI, Greenberg NH, Macdonald MM, Abbott BJ. Modified protocol for the testing of new synthetics in the L1210 lymphoid leukemia murine model in the DR&D program, DCT, NCI. Natl Cancer Inst Monogr 1977;45:151–3
- Gorayski P, Burmeister B, Foote M. Radiotherapy for cutaneous melanoma: Current and future applications. Future Oncol 2015;11:525–34
- Triantopoulou S, Efstathopoulos E, Platoni K, Uzunoglou N, Kelekis N, Kouloulias V. Radiotherapy in conjunction with superficial and intracavitary hyperthermia for the treatment of solid tumors: Survival and thermal parameters. Clin Transl Oncol 2013;15:95–105
- Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol 2012;2:116
- Rodel F, Frey B, Multhoff G, Gaipl U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett 2015;356:105–13
- Orth M, Lauber K, Niyazi M, Friedl AA, Li M, Maihofer C, et al. Current concepts in clinical radiation oncology. Radiat Environ Biophys 2014;53:1–29
- Anvekar RA, Asciolla JJ, Lopez-Rivera E, Floros KV, Izadmehr S, Elkholi R, et al. Sensitization to the mitochondrial pathway of apoptosis augments melanoma tumor cell responses to conventional chemotherapeutic regimens. Cell Death Dis 2012;3:e420
- Pierce RH, Campbell JS, Pai SI, Brody JD, Kohrt HE. In-situ tumor vaccination: Bringing the fight to the tumor. Hum Vaccin Immunother 2015;11:1901–9
- Eggermont AM, Maio M, Robert C. Immune checkpoint inhibitors in melanoma provide the cornerstones for curative therapies. Semin Oncol 2015;42:429–35
- Barker CA, Postow MA. Combinations of radiation therapy and immunotherapy for melanoma: A review of clinical outcomes. Int J Radiat Oncol Biol Phys 2014;88:986–97
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–68
- Schildkopf P, Frey B, Mantel F, Ott OJ, Weiss EM, Sieber R, et al. Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem Biophys Res Commun 2010;391:1014–20
- Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 2015;41:742–53
- Mantel F, Frey B, Haslinger S, Schildkopf P, Sieber R, Ott OJ, et al. Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenther Onkol 2010;186:587–99
- Meggyeshazi N, Andocs G, Balogh L, Balla P, Kiszner G, Teleki I, et al. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther Onkol 2014;190:815–22
- Ullrich E, Bonmort M, Mignot G, Kroemer G, Zitvogel L. Tumor stress, cell death and the ensuing immune response. Cell Death Differ 2008;15:21–8
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res 2005;65:5238–47
- Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, et al. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol 2006;290:C990–9
- Al-Mayah A, Bright S, Chapman K, Irons S, Luo P, Carter D, et al. The non-targeted effects of radiation are perpetuated by exosomes. Mutat Res 2015;772:38–45
- Yang Y, Chen Y, Zhang F, Zhao Q and Zhong H. Increased anti-tumour activity by exosomes derived from doxorubicin-treated tumour cells via heat stress. Int J Hyperthermia 2015;31:498–506
- Breuninger S, Erl J, Knape C, Gunther S, Regel I, Rödel F, et al. Quantitative analysis of liposomal heat shock protein 70 (Hsp70) in the blood of tumor patients using a novel LipHsp70 ELISA. Clin Cell Immunol 2014;5:1–10
- Binder DC, Fu YX, Weichselbaum RR. Radiotherapy and immune checkpoint blockade: Potential interactions and future directions. Trends Mol Med 2015;21:463–5
- Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia 2015;31:609–14
- Hurwitz M, Stauffer P. Hyperthermia, radiation and chemotherapy: The role of heat in multidisciplinary cancer care. Semin Oncol 2014;41:714–29
- Kelleher DK, Vaupel P. No sustained improvement in tumor oxygenation after localized mild hyperthermia. Adv Exp Med Biol 2010;662:393–8
- Frey B, Rubner Y, Kulzer L, Werthmoller N, Weiss EM, Fietkau R, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 2014;63:29–36
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 2009;6:e10
- Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol 2012;22:319–26
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol 2015;16:795–803
- Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–7
- Frey B, Gaipl US. Radio-immunotherapy: The focused beam expands. Lancet Oncol 2015;16:742–3
