Abstract
Purpose: Histological response assessment following neoadjuvant treatment can help identify patients at a higher risk for systemic disease progression. Our goal was to evaluate whether mitotic count and the amount of viable tumour following neoadjuvant isolated limb perfusion (ILP) for primary, locally advanced, non-metastatic, high-grade extremity soft tissue sarcoma correlate with prognosis. Patients and methods: This study is a retrospective analysis of 61 patients who underwent neoadjuvant ILP followed by surgical resection with curative intent between 2001 and 2011. Non-parametric analyses were carried out with the Mann-Whitney U and the Wilcoxon signed-rank test. Survival curves were calculated with the Kaplan-Meier method and compared with the log-rank test. Results: The median follow-up was 44 months for all patients and 55 months for survivors. The amount of viable tumour after ILP had no correlation with overall (OS) (P = 0.227) or event-free (EFS) (P = 0.238) survival probability. Patients with a low mitotic count after ILP had a significantly higher OS (P < 0.001), EFS (P = 0.002) and post-relapse survival probability (P = 0.030) compared to patients with an intermediate or high mitotic count. Conclusions: The mitotic count following ILP for primary, high-grade, locally advanced, non-metastatic soft tissue sarcoma appears to significantly correlate with prognosis. If these results are validated in a prospective setting, they could provide a rationale for the design of adjuvant systemic chemotherapy trials with the goal of improving the prognosis of patients with an intermediate or high mitotic count after ILP.
Introduction
The wide excision of the primary tumour is the treatment of choice for patients with adult-type, localised, high-grade soft tissue sarcomas [Citation1]. However, locally advanced, high-grade tumours often pose a challenge even for experienced surgeons, as the proximity of the tumour to important anatomical structures – such as nerves, bone and vessels – can make a primary limb-sparing excision with clear margins problematic or even impossible to achieve [Citation2,Citation3]. Neoadjuvant treatment modalities are therefore often needed to improve local control and disease outcome [Citation4].
An effective treatment option for tumours confined in an extremity is hyperthermic isolated limb perfusion (ILP) with tumour necrosis factor-alpha (TNF-α) and melphalan [Citation1,Citation5], a regional neoadjuvant modality enabling limb-sparing surgery in 70–80% of patients with locally advanced tumours previously thought to be candidates for amputation [Citation6]. ILP has, however, no influence on the risk of these patients to develop distant metastases, which is estimated to approximately 50–60% [Citation2,Citation3].
Histological response assessment following a neoadjuvant treatment can help identify which patients are at a higher risk for systemic disease progression [Citation4,Citation7,Citation8]. This has been well documented in patients with osteosarcoma and Ewing sarcoma, where the standardised histological evaluation of the amount of viable tumour after neoadjuvant chemotherapy has proven to be an important prognostic factor [Citation8,Citation9]. However, no standardised approach has been established for the histological response assessment following neoadjuvant treatment in soft tissue sarcoma patients [Citation10,Citation11]. Our group recently showed that the mitotic count and the amount of viable tumour after neoadjuvant systemic chemotherapy correlate with prognosis in patients with primary, localised, high-grade soft tissue sarcomas [Citation12]. To our knowledge, the amount of viable tumour after ILP has been evaluated as a prognostic parameter in one previous study, which could only demonstrate a trend toward an improved overall survival with lower amounts of viable tumour after ILP [Citation10]. Despite the fact that changes in mitotic count have been described after induction chemotherapy in patients with soft tissue sarcomas [Citation13], the prognostic significance of this parameter in evaluating tumour response has been largely ignored [Citation12], with only one small study reporting on an improved prognosis for patients with a low mitotic count after ILP [Citation14].
The objective of this study was to evaluate the prognostic significance of the mitotic count and the amount of viable tumour after neoadjuvant ILP in patients with primary, locally advanced, non-metastatic high-grade soft tissue sarcoma of the extremities.
Patients and methods
Study design
Between 2001 and 2011, 63 consecutive patients with primary, locally advanced, non-metastatic, high-grade soft tissue sarcomas underwent neoadjuvant ILP followed by delayed surgical tumour resection at the Sarcoma Centre Berlin-Brandenburg. Two patients received adjuvant systemic chemotherapy and were excluded from our analysis, leaving 61 patients as the subject of this study. All patients signed an informed consent form at hospital admission allowing the use of anonymised data for research purposes.
Data regarding patient demographics, tumour characteristics, first-line treatment and follow-up were collected prospectively and entered into an electronic database. Further details regarding the amount of viable tumour and the mitotic count before and after treatment were collected retrospectively from pathology reports. A sarcoma pathologist (M.W.), blinded to the clinical characteristics and patients’ outcome, re-examined the surgical specimens of patients with missing data. Survival analysis was based on follow-up data as of May 2013. Follow-up information for patients who had stopped presenting at our outpatient clinic was gathered by contacting the referring physicians.
Neoadjuvant treatment
An interdisciplinary panel made the decisions regarding neoadjuvant treatment based on tumour histology, localisation, and extent, as well as patient performance status and preference. Our protocol regarding ILP has been described previously [Citation6]. In short, the procedure was performed under mild hyperthermia. TNF-α dosage amounted to 1–4 mg for the lower and 1–3 mg for the upper extremity, while melphalan dosage amounted to 10 mg/L perfused tissue for the lower and 13 mg/l perfused tissue for the upper extremity. Surgical tumour resection was performed approximately 6 weeks later.
Pathological assessment of response
All surgical specimens were evaluated by pathologists specialising in bone and soft tissue sarcomas in a standardised manner, according to the technique established for bone sarcomas [Citation15]. The surgical specimens were fixated in 4% formalin solution for 24 h. Following a bisection along their greatest diameter, a longitudinal section and then several transversal sections, according to the size of the tumours, were first obtained. A gross estimation of the residual amount of viable tumour was recorded, taking into consideration areas of cystic degeneration, haemorrhage or fibrosis. An amount of <10% residual viable tumour was classified as ‘low’, while an amount of ≥10% residual viable tumour was classified as ‘high’, analogous to the Salzer-Kuntschik grading system for osteosarcoma patients [Citation15]. The number of mitoses was recorded both in the surgical specimen after ILP and, when available, in the biopsy specimen. Mitoses were counted in at least 10 high-power fields (HPF), which correspond to 3.06 mm2 in our microscopes, at the areas showing the highest tumour activity. A mitotic count of 0–9 mitoses per 10 HPF was classified as ‘low’, a count of 10–19 mitoses per 10 HPF as ‘intermediate’, while a count of ≥20 mitoses per 10 HPF was classified as ‘high’ [Citation16].
Follow-up
As all patients had primary, high-grade soft tissue sarcomas, they received uniform post-operative surveillance recommendations. Routine follow-up included clinical examination and a magnetic resonance imaging (MRI) of the primary tumour site every 3 months for the first 2 years after resection of the primary tumour, followed by 6-month intervals for the 3–5 years, followed by annual examinations. Chest imaging was also performed with conventional radiographs at the same intervals and computed tomography (CT) scans annually for the first 5 years. Local recurrences were defined as new solid lesions in the primary tumour site depicted in the MRI. Pulmonary metastases were defined as new or growing lung lesions. In case of suspicious but unclear findings, a biopsy was performed.
Statistical analysis
Non-parametric analyses were carried out with the Mann-Whitney U and the Wilcoxon signed-rank test. The duration of follow-up and the time to event (local recurrence, metastasis or death) were calculated from the date of diagnostic biopsy. Post-relapse survival probability was defined as the interval between the date of the first event and the date of last follow-up. Receiver operating characteristic (ROC) curves were used to analyse the diagnostic accuracy of the mitotic count after ILP regarding overall and event-free survival. The area under the curve (AUC) values were calculated using a non-parametric distribution assumption. The optimal cut-off value was determined using the Youden index [Citation17]. Survival curves were calculated with the Kaplan-Meier method and compared with the log-rank test. Statistical analyses were performed with the IBM SPSS Statistics for Windows software version 20.0 (IBM Corp., Armonk, NY). All P values are two-sided; a P value < 0.05 was considered significant.
Results
Patient demographics and tumour characteristics are listed in . The tumours were classified as high grade according to the TNM classification, as described in the latest WHO classification of tumours of soft tissue and bone [Citation18]. The median patient age at diagnosis was 57 years (range 17–81 years). The median tumour size at diagnosis was available for 50 patients and amounted to 9 cm (range 3–34 cm). The median tumour size after neoadjuvant treatment, available for 53 patients, was 8 cm (range 0–33 cm; P = 0.044). The median follow-up amounted to 44 months for all patients (range 8–138 months) and 55 months for survivors (range 14–138 months).
Table 1. Patient demographics and tumour characteristics.
All patients underwent surgical resection of the primary tumour following neoadjuvant treatment. Limb-sparing surgery was possible in 58 patients, while an amputation had to be performed in three patients. Surgical margins were clear in 52 patients and microscopically positive in nine patients. 19 patients underwent adjuvant radiation treatment.
The amount of viable tumour after ILP amounted to <10% in 22 patients and ≥10% in 39 patients. The mitotic count prior to ILP was available for 35 patients and amounted to a median of 20/10 HPF (range 0–200/10 HPF, mean 29/10 HPF). The mitotic count after ILP was available for all patients and was significantly lower (median 8/10 HPF, range 0–108/10 HPF, mean 15/10 HPF, P = 0.01).
A local recurrence developed in nine patients after a median time of 16 months (range 4–86 months). Seven of these patients had had clear surgical margins. 27 patients developed distant metastases after a median interval of 12 months (range 2–62 months). At the time of the last follow-up 35 patients were alive without evidence of disease, 5 patients were alive with disease and 21 patients had died of their disease. Overall survival probability (OS) at 2 years was 85% and 59% at 5 years. Event-free survival probability (EFS) at 2 and 5 years amounted to 63% and 49%, respectively.
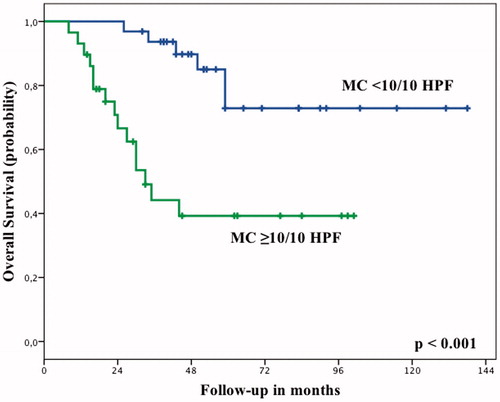
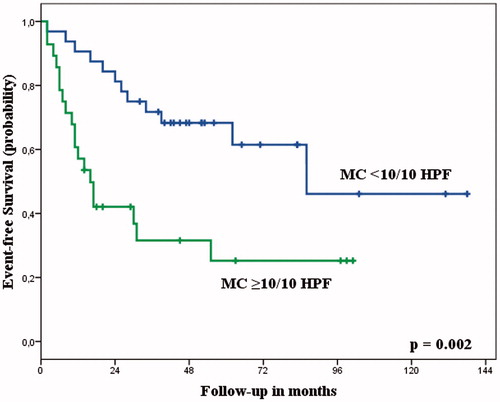
With the number of patients available for this study, the amount of viable tumour after ILP had no correlation with OS or EFS. Patients with <10% viable tumour after ILP had an OS of 66% at 5 years, compared to 56% for patients with ≥10% viable tumour (P = 0.227). EFS at 5 years was 60% compared to 43% (P = 0.238). In this selected group of patients with high-grade tumours the mitotic count at diagnosis also had no statistically significant influence on OS or EFS (), although this might be attributed to a more limited sample size. However, patients with a low mitotic count after ILP had a significantly higher OS (P < 0.001, ) and EFS (P = 0.002, ) compared to patients with an intermediate or high mitotic count (). The ROC curve analysis confirmed that the mitotic count after ILP could predict OS and EFS, with an AUC of 0.738 (95% confidence interval (CI) 0.606–0.870, P = 0.002) and 0.679 (95% CI 0.544–0.814, P = 0.017), respectively. The optimal cut-off value was a mitotic count of 9.5/10 HPF.
Table 2. Mitotic count and survival.
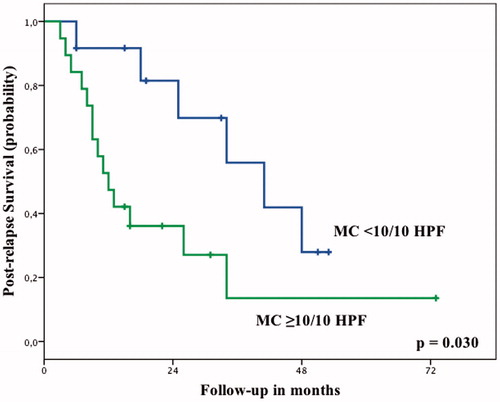
The mitotic count after ILP was also significantly associated with post-relapse survival probability (PRS). After a local or systemic recurrence, patients with a mitotic count <10/10 HPF had a PRS of 82% at 2 years, compared to 36% for patients with a mitotic count ≥10/10 HPF (P = 0.03, ). Expectedly, the amount of viable tumour after treatment did not correlate with PRS. Patients with <10% viable tumour had a PRS of 68% at 2 years, compared to 47% for patients with ≥10% viable tumour (P = 0.295).
In order to address the potential limitations of evaluating outcomes of different prognostic significance, such as local recurrence and distant metastasis, in the variables EFS and PRS we performed separate analyses of metastasis-free survival probability (MFS) and post-metastasis survival probability (PMS). Again, the amount of viable tumour after ILP had no correlation with MFS (P = 0.317) and PMS (P = 0.138). On the other hand, patients with a mitotic count <10/10 HPF had a MFS of 75% at 5 years, compared to 35% for patients with a mitotic count ≥10/10 HPF (P = 0.003), and a PMS of 76% at 2 years, compared to 28% (P = 0.009).
Discussion
ILP with TNF-α and melphalan is an important neoadjuvant treatment modality for soft tissue sarcomas of the extremities, as it can improve local control and facilitate limb-sparing surgery in patients with locally advanced, high-grade tumours [Citation6]. However, these patients are also at a high risk of developing distant metastases [Citation2,Citation3]. Several studies have evaluated the oncological outcome after ILP for locally advanced soft tissue sarcomas [Citation19–21]. Tumour localisation in the upper extremity, tumour multifocality, and previous radiation treatment have been identified as negative prognostic factors for local control, while a large tumour size and a leiomyosarcoma histology have been identified as negative prognostic factors for overall survival probability [Citation19–21].
Although histological response to neoadjuvant treatment is one the most important prognostic factors in patients with osteosarcoma [Citation9] and Ewing sarcoma [Citation8], no validated response assessment system is available for patients with soft tissue sarcomas [Citation1]. Following a recent report from our group showing that the amount of viable tumour and the mitotic count after neoadjuvant systemic chemotherapy correlated with disease-specific and event-free survival in patients with primary, high-grade soft tissue sarcomas [Citation12], we aimed at evaluating the prognostic value of these parameters in patients undergoing neoadjuvant ILP. Inevitably, the decision to include only patients with primary, locally advanced, non-metastatic high-grade tumours who underwent neoadjuvant ILP followed by the delayed surgical resection of the primary tumour and received no adjuvant systemic chemotherapy resulted in the small size of our analysis, which is one of the main limitations of this study, while another is its retrospective nature. On the other hand, these strict inclusion criteria provided us with a highly homogeneous patient population, reducing the influence that confounding factors might have on our findings.
A further limitation of our study is the use of the Salzer-Kuntschik grading system to evaluate the amount of residual viable tumour, which has only been validated in patients with osteosarcomas and Ewing sarcomas. We chose to use this grading system, rather than determining an exact percentage point of the amount of residual viable tumour, as the former represents a well-described system with a clear methodology to allow an estimate of the amount of viable tumour, categorised into six groups, which is easy to reproduce and has at least been validated in bone sarcoma patients. The same approach has been shown to allow a correlation of the amount of viable tumour with the prognosis of patients with high-grade soft tissue sarcomas following neoadjuvant systemic chemotherapy [Citation12].
Contrary to our results in patients following systemic chemotherapy [Citation12], the amount of viable tumour after neoadjuvant ILP had no influence on OS or EFS in this study. However, the mitotic count after ILP was an important predictor not only of OS and EFS, but of PRS as well. To our knowledge, only one previous study has evaluated, until now, the prognostic relevance of the mitotic count following neoadjuvant ILP. In an analysis of 37 patients with locally advanced tumours, Plaat et al. demonstrated that patients who went on to develop distant metastases and patients who died of disease had a significantly higher number of mitoses in the surgical specimen following ILP, compared to patients who did not develop metastases and patients who did not die of disease, respectively [Citation14].
The use of the mitotic count as a prognostic parameter following ILP has the drawback that it is sensitive to delays in fixation, subject to inter-observer variation [Citation22], while it also depends on the size of the microscope field of view [Citation23]. As a result, Ki-67 staining was proposed as an alternative method to evaluate cell proliferation [Citation22], and was shown to have a higher reproducibility than mitotic count [Citation24]. Nonetheless, the mitotic count continues to be one of the parameters employed in the most widely used system to evaluate tumour grade in soft tissue sarcomas [Citation12,Citation25], while it has also been established as one of the prognostic parameters used to identify which patients with localised, operable gastrointestinal stromal tumours have a high risk of developing recurrent disease and should therefore be offered adjuvant systemic treatment [Citation23].
The fact that the mitotic count after ILP correlated with prognosis in the study of Plaat et al. and our study is all the more interesting because ILP is a regional treatment thought to have no influence on overall survival itself [Citation6]. Other prognostic markers following ILP have previously been identified in the literature as well. Our group has demonstrated that the maximal standardised uptake value (SUVmax) after ILP in patients with locally advanced, high-grade extremity soft tissue sarcomas evaluated with PET/CT had a significant correlation with metastasis-free survival probability [Citation3], while Grabellus et al. have shown that patients with a complete histopathological tumour regression following ILP had a trend for an improved overall survival probability compared to patients with less than complete tumour regression [Citation10]. The reasons for these observations remain unclear. One theoretical explanation is that the histological response to neoadjuvant treatment might be indicative of the tumour’s biological aggressiveness, rather than a mere reflection of the treatment effect [Citation3,Citation26]. This theory would also explain the correlation between a low mitotic count after ILP and an improved PRS. It has also been hypothesised that the surgical excision of the primary tumour may result in a stimulation of metastatic disease both in animal models and in cancer patients [Citation27,Citation28]. In this case it would be conceivable that the changes induced in the primary tumour and its microenvironment after ILP might influence the disease outcome in some patients [Citation3], but there is no evidence to support or refute this notion in the literature.
Assuming that the prognostic impact of the mitotic count following ILP can be validated in a separate patient group, a question that will need to be addressed is whether patients with an intermediate or high mitotic count after ILP are good candidates for conventional adjuvant systemic chemotherapy regimens [Citation3]. The results of studies on doxorubicin-based adjuvant treatment plans have been conflicting [Citation1]; however, recent analyses have suggested a limited but statistically significant survival benefit for high-risk patients with high-grade, large, deep-seated tumours [Citation1,Citation29,Citation30]. If an intermediate or high mitotic count after ILP is indicative of a more aggressive disease biology, rather than an inherent tumour resistance to cytotoxic treatment, it would be conceivable that patients with an intermediate or high mitotic count after ILP might also benefit from adjuvant, doxorubicin-based treatment regimens; however, this issue will have to be addressed in future prospective studies [Citation3].
Conclusion
In conclusion, the mitotic count following ILP for primary, high-grade, locally advanced, non-metastatic extremity soft tissue sarcomas appears to be significantly correlated with overall, event-free and post-relapse survival probability. If these results are validated in a prospective setting they could provide a rationale for the design of adjuvant systemic chemotherapy trials with the goal of improving the prognosis of patients with an intermediate or high mitotic count after ILP.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii102–12
- Clarkson P, Ferguson PC. Primary multidisciplinary management of extremity soft tissue sarcomas. Curr Treat Options Oncol 2004;5:451–62
- Andreou D, Boldt H, Pink D, Jobke B, Werner M, Schuler M, et al. Prognostic relevance of 18F-FDG PET uptake in patients with locally advanced, extremity soft tissue sarcomas undergoing neoadjuvant isolated limb perfusion with TNF-alpha and melphalan. Eur J Nucl Med Mol Imaging 2014;41:1076–83
- Eilber FC, Rosen G, Eckardt J, Forscher C, Nelson SD, Selch M, et al. Treatment-induced pathologic necrosis: A predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 2001;19:3203–9
- Jakob J, von Rege I, Weiss C, Hohenberger P. Impact of hyperthermic isolated limb perfusion on tumour oxygenation in soft tissue sarcoma. Int J Hyperthermia 2012;28:591–6
- Andreou D, Fehlberg S, Tiedke C, Niethard M, Tunn PU. Revolutionizing the treatment of locally advanced extremity soft tissue sarcomas: A review on TNFα-based isolated limb perfusion. Eur Surg 2009;41:176–88
- Schmidt RA, Conrad EU III, Collins C, Rabinovitch P, Finney A. Measurement and prediction of the short-term response of soft tissue sarcomas to chemotherapy. Cancer 1993;72:2593–601
- Picci P, Rougraff BT, Bacci G, Neff JR, Sangiorgi L, Cazzola A, et al. Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing's sarcoma of the extremities. J Clin Oncol 1993;11:1763–9
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002;20:776–90
- Grabellus F, Kraft C, Sheu-Grabellus SY, Bauer S, Podleska LE, Lauenstein TC, et al. Tumor vascularization and histopathologic regression of soft tissue sarcomas treated with isolated limb perfusion with TNF-alpha and melphalan. J Surg Oncol 2011;103:371–9
- Lucas DR, Kshirsagar MP, Biermann JS, Hamre MR, Thomas DG, Schuetze SM, et al. Histologic alterations from neoadjuvant chemotherapy in high-grade extremity soft tissue sarcoma: Clinicopathological correlation. Oncologist 2008;13:451–8
- Andreou D, Werner M, Pink D, Traub F, Schuler M, Gosheger G, et al. Prognostic relevance of the mitotic count and the amount of viable tumour after neoadjuvant chemotherapy for primary, localised, high-grade soft tissue sarcoma. Br J Cancer 2015;112:455–60
- Coindre JM, Nguyen BB, Goussot JF, de Mascarel I, Maree D, de Mascarel A, et al. Modifications histologiques aprés chimiothérapie des sarcomes des tissus mous de l'adulte [Histological changes after chemotherapy of soft tissue sarcomas in the adult]. Ann de Pathol 1985;5:95–9
- Plaat BE, Molenaar WM, Mastik MF, Koudstaal J, van den Berg E, Koops HS, et al. Hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan in patients with locally advanced soft tissue sarcomas: Treatment response and clinical outcome related to changes in proliferation and apoptosis. Clin Cancer Res 1999;5:1650–7
- Salzer-Kuntschik M, Delling G, Beron G, Sigmund R. Morphological grades of regression in osteosarcoma after polychemotherapy – study COSS 80. J Cancer Res Clin Oncol 1983;106:S21–4
- Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-tissue sarcomas of adults: Study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984;33:37–42
- Perkins NJ, Schisterman EF. The inconsistency of ‘optimal’ cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670–5
- Fletcher C, Bridge J, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone. Lyon: International Agency for Research on Cancer; 2013
- Deroose JP, Eggermont AM, van Geel AN, Burger JW, den Bakker MA, de Wilt JH, et al. Long-term results of tumor necrosis factor alpha- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. J Clin Oncol 2011;29:4036–44
- Jakob J, Tunn PU, Hayes AJ, Pilz LR, Nowak K, Hohenberger P. Oncological outcome of primary non-metastatic soft tissue sarcoma treated by neoadjuvant isolated limb perfusion and tumor resection. J Surg Oncol 2014;109:786–90
- Pennacchioli E, Deraco M, Mariani L, Fiore M, Mussi C, Collini P, et al. Advanced extremity soft tissue sarcoma: Prognostic effect of isolated limb perfusion in a series of 88 patients treated at a single institution. Ann Surg Oncol 2007;14:553–9
- Daugaard S, von Glabbeke M, Schiodt T, Mouridsen HT. Histopathological grade and response to chemotherapy in advanced soft tissue sarcomas. Eur J Cancer 1993;29A:811–13
- Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265–74
- Hasegawa T, Yamamoto S, Nojima T, Hirose T, Nikaido T, Yamashiro K, et al. Validity and reproducibility of histologic diagnosis and grading for adult soft-tissue sarcomas. Hum Pathol 2002;33:111–15
- Deyrup AT, Weiss SW. Grading of soft tissue sarcomas: The challenge of providing precise information in an imprecise world. Histopathology 2006;48:42–50
- Huth JF, Mirra JJ, Eilber FR. Assessment of in vivo response to preoperative chemotherapy and radiation therapy as a predictor of survival in patients with soft-tissue sarcoma. Am J Clin Oncol 1985;8:497–503
- Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID. The effects of surgery on tumor growth: A century of investigations. Ann Oncol 2008;19:1821–8
- Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis 2005;26:513–23
- Italiano A, Delva F, Mathoulin-Pelissier S, Le Cesne A, Bonvalot S, Terrier P, et al. Effect of adjuvant chemotherapy on survival in FNCLCC grade 3 soft tissue sarcomas: A multivariate analysis of the French Sarcoma Group Database. Ann Oncol 2010;21:2436–41
- Woll PJ, Reichardt P, Le Cesne A, Bonvalot S, Azzarelli A, Hoekstra HJ, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): A multicentre randomised controlled trial. Lancet Oncol 2012;13:1045–54