Abstract
Purpose: Radiofrequency ablation (RFA) and microwave ablation (MWA) are the two main percutaneous techniques for the treatment of unresectable hepatocellular carcinoma (HCC). However, to date, studies comparing the two therapies have provided discordant results. The aim of this meta-analysis is to evaluate the efficacy and safety of the two treatments for HCC patients. Materials and methods: A computerised bibliographic search was performed on PubMed/MEDLINE, Embase, Google Scholar and Cochrane library databases. The rates of complete response (CR), local recurrence (LRR), 3-year survival (SR) and major complications were compared between the two treatment groups by using the Mantel-Haenszel test in cases of low heterogeneity or the DerSimonian and Laird test in cases of high heterogeneity. Sources of heterogeneity were investigated using subgroup analyses. In order to confirm our finding, sensitivity analysis was performed restricting the analysis to high-quality studies. Results: One randomised controlled trial (RCT) and six retrospective studies with 774 patients were included in the meta-analysis. A non-significant trend of higher CR rates in the patients treated with MWA was found (odds ratio (OR) = 1.12, 95% confidence interval (CI) 0.67–1.88, p = 0.67]. Overall LRR was similar between the two treatment groups (OR 1.01, 95% CI 0.53–1.87, p = 0.98) but MWA outperformed RFA in cases of larger nodules (OR 0.46, 95% CI 0.24–0.89, p = 0.02). 3-year SR was higher after RFA without statistically significant difference (OR 0.95, 95% CI 0.58–1.57, p = 0.85). Major complications were more frequent, although not significantly, in MWA patients (OR 1.63, 95% CI 0.88–3.03, p = 0.12). Conclusions: Our results indicate a similar efficacy between the two percutaneous techniques with an apparent superiority of MWA in larger neoplasms.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most commonly occurring type of cancer and the leading cause of mortality in cirrhotic patients [Citation1].
Nowadays, 30–60% of HCC patients in developed countries are suitable for curative therapies, such as surgical or ablative treatments, due to the recent improvement in diagnosis and the screening programmes in cirrhotics [Citation1].
In recent years imaging-guided ablative therapies have gained a fundamental role in the treatment of HCC. Among them, percutaneous radiofrequency ablation (RFA) has become the standard of care for unresectable early HCCs and has been even found to be competitive with surgery in the case of single nodules less than 2 cm [Citation2,Citation3].
Meanwhile, with the advancement of microwave technology and the development of the cooled electrode, percutaneous microwave ablation (MWA) is emerging as a valuable alternative to RFA for thermal destruction of HCC [Citation4,Citation5]. The main features of MWA technology compared with other thermal ablation technologies include consistently higher intratumoural temperatures, larger tumour ablation volumes, faster ablation times, and an improved convection profile. As a result, the advantage of MWA over RFA is that treatment outcome is less affected by vessels in proximity to the tumour (heat-sink effect) [Citation6].
Despite the promising results of MWA reported in the aforementioned studies, little is known about its efficacy compared to RFA. A recent meta-analysis found that the two techniques are equally effective, but the reliability of these results is impaired by the inclusion of low-quality reports such as congress abstracts and duplicate studies [Citation7]. Therefore, robust data on the comparison between RFA and MWA is still lacking.
The aim of this meta-analysis is to evaluate the efficacy and safety of the two treatments for HCC patients. Primary outcome was the local recurrence rate (LRR) registered during the follow-up. Secondary outcomes were tumour response, 3-year survival rate (SR) and incidence of major complications.
Methods
This meta-analysis is performed following indications described in the Cochrane Handbook [Citation8] and is conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [Citation9].
Search strategy and selection criteria
A computerised bibliographic search was performed on PubMed/MEDLINE, Embase, Google Scholar, and the Cochrane library databases using the following key words: ‘radiofrequency ablation’, ‘microwave ablation’, ‘HCC’, ‘hepatocellular carcinoma’ and ‘liver cancer’. Eligible studies were randomised controlled trials (RCTs), prospective or retrospective cohort and case-control studies comparing percutaneous RFA and MWA in human patients until July 2015. The search was restricted to English-language articles. Studies were excluded if they had not compared data between the two treatments. Case reports and abstracts or studies with insufficient data were also excluded. Studies included were selected independently by two investigators (AF and MDM). Disagreements were solved by discussion and following a third opinion (NM).
The quality of the studies included was assessed by the authors independently according to the Cochrane Collaboration’s tool for assessing the risk of bias [Citation8] for RCTs and the Newcastle–Ottawa scale for observational studies [Citation10].
Statistical analysis
Data of LRR, SR, CR rate and toxicity (expressed as severe adverse events rate) were pooled and analysed in terms of odds ratio (OR, 95% confidence intervals). Comparisons between the two treatment groups across all the studies included were performed by using the Mantel-Haenszel test for fixed-effects models [Citation11] (in case of low heterogeneity) or the DerSimonian and Laird test for random-effects models [Citation12] (in case of high heterogeneity).
Heterogeneity between estimates was assessed by means of Cochrane’s chi-square test, with the significance threshold settled at 0.10, and I2 statistic, with a value of >50% being suggestive of significant heterogeneity [Citation13].
Between-study sources of heterogeneity were investigated using subgroup analyses, by stratifying original estimates according to study characteristics. Again, in these analyses a p-value <0.10 was considered significant, due to the low power of the tests and the small number of studies included.
Publication biases were assessed using funnel plots visually and by performing Begg and Mazumdar’s test.
In order to confirm our finding, sensitivity analysis was finally performed restricting the analysis to high-quality studies.
All calculations were performed using Review Manager 5.0 (Cochrane Informatics).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study formal consent is not required.
Results
Literature search
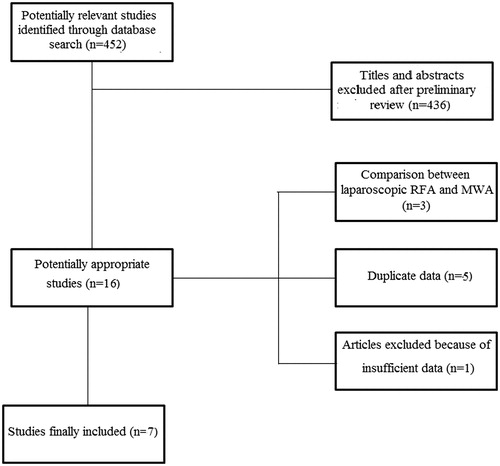
shows the flow chart of the search strategy conducted in this meta-analysis.
Initially, we identified 452 potentially relevant studies. After a preliminary review, 436 papers were excluded because they were animal studies, case reports, comment letters or descriptive reviews.
Among 16 potentially appropriate articles, we excluded three studies including patients treated with laparoscopic RFA or MWA [Citation14–16], and one because of insufficient data [Citation17].
Three studies were published by the same group with overlapping recruitment periods [Citation18–20]. Among them, the two studies with the lower number of patients and incomplete data were excluded in order to overcome the risk of duplicate data [Citation18,Citation19].
Four series were based on data published by the same Chinese group enrolling patients in two partially overlapping periods [Citation21–24]. The same criterion used before was adopted to eliminate duplicate results [Citation21,Citation23,Citation24].
Finally, seven studies with 774 patients were included in the meta-analysis [Citation20,Citation22,Citation25–29].
Characteristics of studies included
Main characteristics of studies included are reported in .
Table 1. Characteristics of the studies included.
The recruitment period ranged from 1997 to 2013. One study was an RCT [Citation25] and six were retrospective case-control studies [Citation20,Citation22,Citation26–29]. Studies included were conducted mostly in Asia [Citation20,Citation22,Citation25–27].
In all the studies except for that by Ohmoto et al. [Citation20], patients were within Child-Pugh B score and mean tumour size ranged between 1.6 and 2.9 cm.
None of the studies reported statistically significant differences in baseline demographic, clinical and tumoural parameters between the two treatment groups. Three observational studies [Citation20,Citation26,Citation27] were considered high quality, whereas all the other reports were deemed of moderate quality.
More details on the methodological characteristics and quality of articles included are shown in Supplementary Table 1.
Complete response
Tumour response was evaluated in six studies [Citation22,Citation25–29]. In all the studies included, CR was defined as absence of residual viable tumour in the treated nodules.
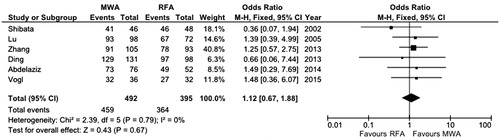
The meta-analysis performed by means of fixed-effect model found a non-significant trend of higher CR rates in patients treated with MWA (OR 1.12, 95% CI 0.67–1.88, p = 0.67) (). No evidence of heterogeneity was found (χ2 = 2.39, df = 5, p = 0.79, I2 = 0%).
Figure 2. Forest plot of complete response rate. df, degrees of freedom; M-H, Mantel-Haenszel; MWA, microwave ablation; RFA, radiofrequency ablation.
No significant publication bias was found either by means of visual examination of funnel plot (Supplementary Figure 1) or by means of Begg and Mazumdar’s test (p = 0.29).
In order to further confirm these findings, sensitivity analysis was performed with two different subgroups analyses. At first, since one of the studies included was a RCT [Citation25], the OR of CR was separately calculated for the RCT and for the retrospective studies, and in both cases did not result in significance (OR 0.36, 95% CI 0.07–1.94, p = 0.23 and OR 1.29, 95% CI 0.74–2.24, p = 0.37, respectively).
Second, articles with lower quality data [Citation22,Citation25,Citation28,Citation29] were eliminated from the analysis and the resulting OR remained not significantly in favour of MWA (OR 1.17, 95% CI 0.56–2.47, p = 0.68) with no evidence of heterogeneity (χ2 = 0.24, df = 1, p = 0.63, I2 = 0%).
Local recurrence rate
All the studies included reported LRR. Meta-analysis of such an outcome did not find any differences between the two treatment groups (OR 1.01, 95% CI 0.53–1.87, p = 0.98) ().
Figure 3. Forest plot of local recurrence rate. df, degrees of freedom; MWA, microwave ablation; RFA, radiofrequency ablation.
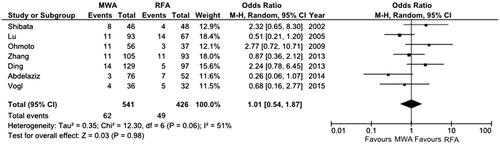
The overall OR was computed by means of the DerSimonian-Laird test because of the high grade of heterogeneity found among the studies included (χ2 = 12.3, df = 6, p = 0.06, I2 = 51%) ().
The Funnel plot and Begg and Mazumdar’s test (p = 0.59) did not show any evidences of publication bias (Supplementary Figure 2).
In order to assess the influence of lower quality studies on the final result and to explore the sources of heterogeneity, OR was re-calculated considering only high quality papers. After performing this kind of sensitivity analysis, OR remained non-significant (OR 1.57, 95% CI 0.76–3.26, p = 0.23) with a lower grade of heterogeneity (χ2 = 2.79, df = 2, p = 0.25, I2 = 28%).
Unfortunately, accurate stratification of results according to tumour stage was not possible due to the low number of studies. However, when the meta-analysis was restricted to the three studies enrolling patients with high tumour burden [Citation22,Citation28,Citation29], MWA significantly outperformed RFA (OR 0.46, 95% CI 0.24–0.89, p = 0.02) with no evidence of heterogeneity (χ2 = 0.93, df = 2, p = 0.63, I2 = 0%) (Supplementary Figure 3).
Overall survival
Data on 3-year SR was available in six studies [Citation20,Citation22,Citation26–29].
A high grade of heterogeneity was found (χ2 = 11.2, df = 5, p = 0.05, I2 = 55%), hence the random-effect model was performed. Overall OR was in favour of RFA without a statistically significant difference (OR 0.95, 95% CI 0.58–1.57, p = 0.85) (). No publication bias was found (p = 0.34, Supplementary Figure 4).
Figure 4. Forest plot of overall survival rate at 3 years. df, degrees of freedom; MWA, Microwave ablation; RFA, Radiofrequency ablation.
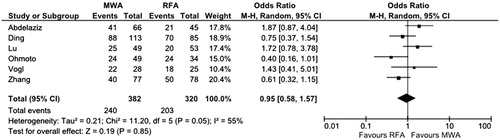
Sensitivity analysis in high quality studies [Citation20,Citation26,Citation27] confirmed the aforementioned non-significant trend in favour of RFA (OR 0.78, 95% CI 0.46–2.16, p = 0.46) with no evidence of heterogeneity (χ2 = 1.13, df = 2, p = 0.57, I2 = 0%).
Major complications
All the studies included reported information about major complications that occurred within 1 month after the procedure.
The rate of major complications was higher after MWA, although without reaching the significance threshold (OR 1.63, 95% CI 0.88–3.03, p = 0.12) with low heterogeneity among studies found, mainly because of a single outlier study [Citation28] (χ2 = 8.36, df = 6, p = 0.12, I2 = 28%) (Supplementary Figure 5).
A visualisation of funnel plot (Supplementary Figure 6) and Begg and Mazumdar’s test (p = 0.41) did not find any source of publication bias.
Discussion
Local ablation is considered the first-line treatment option for early HCC patients not suitable for surgical therapies [Citation1]. Over the past 25 years several methods for chemical or thermal tumour ablation have been developed and clinically tested.
The seminal technique was percutaneous ethanol injection (PEI), which induces coagulative necrosis of the lesion [Citation6]. Subsequently, thermal ablative therapies emerged, including RFA, MWA and laser ablation.
Although both percutaneous RFA and MWA have been commonly used for the treatment of HCC, the differences between the two modalities have not been clearly documented and few studies aimed at the comparison between the two techniques have been published so far. In a recent meta-analysis, Chinnaratha et al. [Citation7] found that RFA and MWA are equally effective in HCC patients with a potential superiority of MWA in terms of local recurrence in larger neoplasms. However, the results of this paper should be interpreted with caution as low-quality studies and even congress abstracts were included; furthermore, all the reports included had been conducted in Asia, thus raising some concerns on the applicability of their findings in the West [Citation7].
On the basis of these considerations and of the recent publication of two additional studies conducted in areas other than Eastern Asia, we decided to publish the current meta-analysis in order to provide a robust and up-to-date overview of the available data in this field.
A total of seven studies, with one a RCT with 774 patients, were included in this meta-analysis. As for the primary outcome, i.e. local recurrence rate, no significant difference was found overall (OR 1.01, 95% CI 0.53–1.87, p = 0.98). The high grade of heterogeneity (I2 = 51%, p = 0.06) decreased when the analysis was restricted solely to the high-quality studies [Citation20,Citation26,Citation27].
Although accurate stratification of results according to tumour stage was not possible, when only the studies enrolling patients with larger tumour size [Citation22,Citation28,Citation29] were considered, MWA significantly outperformed RFA (OR 0.46, 95% CI 0.24–0.89, p = 0.02) with no evidence of heterogeneity (I2 = 0%, p = 0.63). This result may be interpreted on the basis of the well-known property of MWA, which is the ability to induce higher intratumoural temperatures, larger tumour ablation volumes, faster ablation times, and an improved convection profile [Citation6,Citation30]. Therefore, the apparent superiority of MWA over RFA in treating larger nodules is not surprising.
MWA was found to provide a higher CR rate than RFA, although such a difference did not result in statistical significance (p = 0.67). As recently proved, RFA is able to provide complete ablation rates >95% in selected series [Citation31], hence the superiority, although not significant, of MWA according to tumour response is an important proof of effectiveness of this technique.
Despite the better CR and lower LRR of MWA, overall survival estimated at 3 years was higher after percutaneous RFA, even if not significantly (OR 0.95, 95% CI 0.58–1.57, p = 0.85).
In order to understand these results, many factors other than response to treatment have to be considered. RFA is a safer technique (see below) with lower rates of severe adverse events; moreover, as recently demonstrated by our group, local recurrences are generally easy to treat and do not severely impair post-recurrence survival [Citation32]. Therefore, the proved superiority of MWA in larger neoplasms does not seem to lead automatically to better survival outcomes.
As mentioned above, the rate of major complications was higher, although not significantly, after MWA. This result was reported in almost all the studies included, with the only exception being the paper by Abdelaziz et al. [Citation28], which was responsible for both the failure to reach the OR significance threshold and for the moderate heterogeneity observed (I2 = 28%, p = 0.12). The more favourable safety profile of RFA can be explained in light of the broader necrotic area obtained after MWA with an increased risk of vessel damage or liver abscesses [Citation30,Citation33].
There are some limitations to our study. First, we analysed both prospective and retrospective studies with no standard randomisation which may introduce patient selection bias. However, as described in , no significant difference according to baseline characteristics between the two treatment groups was detectable in any of the studies included. Second, the lack of standardisation of MWA equipment restricts direct comparison of the studies included in the meta-analysis, and a subgroup analysis based on equipment was not possible due to the small numbers within each treatment group. Moreover, the current meta-analysis includes many studies conducted in a time period when microwave ablation devices and experience were not optimised at all. Therefore, further RCTs and cohort studies are needed in order to confirm our findings. Third, the number of reports included is small. This apparent drawback of our paper is due to the exclusion of duplicate studies or reports with incomplete data. However, as a consequence of the restrictive inclusion criteria adopted in our meta-analysis, the quality level of the manuscript reviewed was mostly high, thus increasing the robustness of our findings. Fourth, because of the small number of studies, an accurate stratification of outcomes according to lesion size was not possible. However, LRR analysis was separately performed in two different groups of studies on the basis of mean baseline tumour burden.
Despite these limitations, our study has a number of strengths. It is the more comprehensive and up-to-date meta-analysis comparing the two main percutaneous thermal ablation techniques for primary liver tumours. Moreover, any possible sources of heterogeneity that could have influenced the final results were explored by means of appropriate statistical tools and all the findings were confirmed by performing sensitivity analysis.
In conclusion, the current meta-analysis shows that percutaneous MWA provides competitive if not superior results with respect to RFA in terms of CR and recurrence rate, particularly in larger tumours. Despite these findings, overall survival and safety profile were in favour, although not significantly, of RFA. Further RCTs are needed in order to validate these results.
Declaration of interest
The authors report no conflicts of interest. A.F. designed the study and performed the statistical analysis, A.F. and M.DiM. collected the data, N.M. revised the manuscript. All the authors approved the final draft submitted. The authors alone are responsible for the content and writing of the paper.
References
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118–27
- Nishikawa H, Kimura T, Kita R, Osaki Y. Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia 2013;29:558–68
- Hocquelet A, Balageas P, Laurent C, Blanc JF, Frulio N, Salut C, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: A study of 281 Western patients. Int J Hyperthermia 2015;31:749–57
- Li M, Yu X, Liang P, Dong B, Liu F. Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia 2015;31:579–87
- Livraghi T, Meloni F, Solbiati L, Zanus G, Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: Results of a multicenter study. Cardiovasc Intervent Radiol 2012;35:868–74
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43–58
- Chinnaratha MA, Chuang MA, Fraser RJ, Woodman RJ, Wigg AJ. Percutaneous thermal ablation for primary hepatocellular carcinoma: A systematic review and meta-analysis. J Gastroenterol Hepatol 2015; in press
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151:264–9, W64
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm works (accessed 6 July 2015)
- Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986;42:311–23
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60
- Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: Safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol 2011;104:822–9
- Cillo U, Vitale A, Dupuis D, Corso S, Neri D, D'Amico F, et al. Laparoscopic ablation of hepatocellular carcinoma in cirrhotic patients unsuitable for liver resection or percutaneous treatment: A cohort study. PLoS One 2013;8:e57249
- Iida H, Aihara T, Ikuta S, Yamanaka N. A comparative study of therapeutic effect between laparoscopic microwave coagulation and laparoscopic radiofrequency ablation. Hepatogastroenterology 2013;60(124):662–5
- Izumi N, Asahina Y, Noguchi O, Uchihara M, Kanazawa N, Itakura J, et al. Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation. Cancer 2001;91:949–56
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Thermal ablation therapy for hepatocellular carcinoma: Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy. Hepatogastroenterology 2006;53(71):651–54
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Radiofrequency ablation versus percutaneous microwave coagulation therapy for small hepatocellular carcinomas: A retrospective comparative study. Hepatogastroenterology 2007;54(76):985–9
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009;24:223–7
- Xu HX, Xie XY, Lu MD, Chen JW, Yin XY, Xu ZF, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol 2004;59:53–61
- Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterol 2005;40:1054–60
- Yin XY, Xie XY, Lu MD, Xu HX, Xu ZF, Kuang M, et al. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: Long-term outcome and prognostic factors. Cancer 2009;115:1914–23
- Kuang M, Xie XY, Huang C, Wang Y, Lin MX, Xu ZF, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg 2011;15:2165–71
- Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331–7
- Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379–84
- Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One 2013;8:e76119
- Abdelaziz AO, Nabeel MM, Elbaz TM, Shousha HI, Hassan EM, Mahmoud SH, et al. Microwave ablation versus transarterial chemoembolization in large hepatocellular carcinoma: Prospective analysis. Scand J Gastroenterol 2015;50:479–84
- Vogl TJ, Farshid P, Naguib NN, Zangos S, Bodelle B, Paul J, et al. Ablation therapy of hepatocellular carcinoma: A comparative study between radiofrequency and microwave ablation. Abdom Imaging 2015;40:1829–37
- Farina L, Weiss N, Nissenbaum Y, Cavagnaro M, Lopresto V, Pinto R, et al. Characterisation of tissue shrinkage during microwave thermal ablation. Int J Hyperthermia 2014;30:419–28
- Facciorusso A, Del Prete V, Antonino M, Neve V, Crucinio N, Di Leo A, et al. Serum ferritin as a new prognostic factor in hepatocellular carcinoma patients treated with radiofrequency ablation. J Gastroenterol Hepatol 2014;29:1905–10
- Facciorusso A, Del Prete V, Antonino M, Crucinio N, Neve V, Di Leo A, et al. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig Liver Dis 2014;46:1014–19
- Cavagnaro M, Amabile C, Cassarino S, Tosoratti N, Pinto R, Lopresto V. Influence of the target tissue size on the shape of ex vivo microwave ablation zones. Int J Hyperthermia 2015;31:48–57