Abstract
Purpose: The aim of this study was to evaluate the outcomes of loco-regional hyperthermia (HT) with radiotherapy (RT) and/or chemotherapy (CT) in elderly patients with muscle-invasive bladder cancers (MIBC). Material and methods: Twenty consecutive MIBC patients were treated with HTRT (n = 8) or HTCTRT (n = 12) following transurethral resection of their bladder tumours. Weekly HT was administered prior to RT to a mean temperature of 40.6–42.7 °C for 60 min. A mean RT dose of 54.6 Gy (SD ± 4.2) was delivered. Single-agent cisplatin (n = 2) or carboplatin (n = 10) was used in HTCTRT patients. Results: The median age was 81 years. HTRT patients received a mean RT dose of 51.0 Gy compared to 57.1 Gy with HTCTRT (p < 0.001) in a shorter overall treatment time (OTT) (30.8 ± 6.9 versus 43.9 ± 4.0 days, p < 0.001). All HTRT patients had long-term local disease control, while 41.6% of HTCTRT recurred during follow-up. None of the HTRT patients experienced grade III/IV acute and late toxicities, while these were evident in two and one HTCTRT patients respectively. Taken together, the 3-year bladder preservation, local disease-free survival, cause-specific survival and overall survival were 86.6%, 60.7%, 55% and 39.5% respectively. Even though the mean biological effective dose (BED) for both groups was similar (57.8 Gy15), the thermo-radiobiological BED estimated from HT-induced reduction of α/β was significantly higher for HTRT patients (91 ± 4.4 versus 85.8 ± 4.3 Gy3, p = 0.018). Conclusions: Thermal radiosensitisation with consequent reduction in α/β results in a higher thermo-radiobiological BED with a relatively higher RT dose/fraction and shorter OTT. This translates into a favourable outcome in elderly MIBC patients. Any benefit of CT in these patients needs further investigation.
Introduction
Globally there are 0.46 million cases of cancer of the bladder and these are projected to nearly double to around 0.80 million by 2035 [Citation1]. Around 66.4% of the patients are aged 65 or over and by 2035 this is expected to increase to 73.5%. The anticipated rise of elderly patients from 0.31 million in 2015 to 0.59 million by 2035, represents a steep increase of 89.7% in the coming decades. Radical cystectomy with urinary diversion is still considered the standard treatment option for muscle-invasive bladder cancers (MIBC). However, as the population ages, it is likely that there will be an increasing number of elderly patients unfit for such extensive surgical interventions due to associated co-morbidities [Citation2–6]. Radical cystectomy in octogenarians may not even be associated with an improved overall survival [Citation7]. Thus, the demand for bladder-sparing approaches in MIBC is likely to increase in the near future and needs to be explored further. Traditionally these strategies have evolved with the use of chemoradiotherapy (CTRT), and offer a potential cure for those unfit for surgery [Citation8–10]. However, in elderly patients, the significant acute and late morbidities associated with chemotherapy (CT) could be a deciding factor in treatment compliance and resulting outcomes [Citation9,Citation11,Citation12].
Hyperthermia (HT) at 39–43 °C is known to be a potent radio- and chemosensitiser [Citation13]. It has been shown to be effective for non-muscle-invasive bladder cancers when applied intravesically with instillation of CT agents, such as, mitomycin C [Citation14,Citation15]. In MIBC, HT has been used with radiotherapy (RT) alone either as a preoperative or definitive treatment [Citation16,Citation17], or with CTRT, both resulting in positive outcomes [Citation18–20]. Thermal radiosensitisation has been primarily attributed to reoxygenation, selective killing of radioresistant hypoxic tumour cells, and inhibition of repair of radiation-induced DNA damage [Citation13]. This influences the linear-quadratic (L-Q) parameters of the clonogenic cell survival curves. At 41 °C for 1 h (as for clinical treatments), HT results in an increase in the β-component of the cell kill of the L-Q model, leading to a reduction in the α/β ratio [Citation21]. This could have implications for a higher RT dose/fraction in conjunction with HT.
We report our initial results with loco-regional HT along with either RT alone or CTRT in elderly patients with MIBC who were medically unfit to be considered for radical surgical procedures or had refused surgery. The outcomes observed for both HTRT and HTCTRT are examined in line with the thermo-radiobiological implications of a combination of HT and RT, to derive an optimal approach for the future management of MIBC with HT and RT or CTRT, especially in elderly patients.
Materials and methods
Patient selection
Between January 2011 and April 2015, 20 consecutive elderly patients with MIBC (≥65 years) who had co-morbidities or had declined cystectomy were considered for a bladder preserving treatment using HT along with RT or CTRT. A detailed medical work-up was undertaken prior to allocation to either of these bladder preservation strategies. This included haematology, serum biochemistry, creatinine clearance, urine cytology, uroflowmetry, post void residual urine, cystoscopy, contrast enhanced computed tomography (CECT) of the thorax, and CECT or magnetic resonance imaging of the abdomen and pelvis. All patients underwent transurethral resection of bladder tumour (TUR-BT) with tumour mapping under general anaesthesia. Resected specimens were subjected to histopathological evaluation. Patients were jointly reviewed at the multidisciplinary urology tumour board and those unfit for surgery or who refused surgery were offered HT along with RT or CTRT. Patients with associated pre-existing renal, cardiovascular or haematological conditions and deemed unfit for CT by the medical oncologists were considered for HTRT alone.
Radiotherapy
Target volumes (gross, clinical and planning) were delineated on the radiotherapy treatment CT scans as per the International Commission on Radiation Units and Measurements (ICRU) reports 50 and 62 [Citation22,Citation23]. The gross target volume (GTV) included all macroscopic tumour tissue visible on CT/MRI/cystoscopy, while the clinical target volume (CTV) included the GTV, whole bladder, pelvic lymph nodes, proximal urethra, prostate and prostatic urethra (in men). The planning target volume (PTV) was the CTV expanded by 2–3 cm. Pelvic irradiation was followed by a boost to the GTV. The boost volume included part of, or the entire bladder according to whether the tumour was solitary or multifocal. The whole pelvis was treated with full bladder while the boost was delivered in empty bladder.
HTRT patients with solitary lesions were planned to receive 36 Gy in 12 fractions followed by a boost of 12 Gy in 3 fractions, resulting in a total dose of 48 Gy in 15 fractions. These patients were scheduled to receive 4 fractions of RT per week. Those with multiple lesions received 50 Gy/20 fractions over 4 weeks. The CTV in HTRT patients did not include the entire pelvic lymphatic drainage region, but was limited to the entire bladder with a 2–3 cm margin.
HTCTRT patients were planned to receive 45 Gy/25 fractions over 5 weeks at 1.8 Gy/fraction to the pelvis. For the boost, patients with multifocal tumours received 12.8 Gy in 6 fractions to the GTV, while those with solitary lesions received 13.2 Gy in 6 fractions. In view of the elderly age, all patients were carefully monitored during treatment and any RT dose modifications were performed according to the individual patient’s tolerance and compliance with treatment.
Patients were treated in a supine position assisted by individualised patient moulds (VacFix vacuum cushions, Par Scientific, Odense, Denmark) with minimum photon beam energy of 6 MV and a 3D conformal technique. Portal images (either electronic portal images or cone-beam CT) were taken for all fields during the first three fractions and then weekly.
Estimation of the biological effective dose with or without hyperthermia
In view of the different time–dose–fractionation schedules, the biological effective dose (BED) was computed for each patient as per the L-Q model [Citation24]:
(1)
where, n represents the number of fractions and d the dose/fraction. In view of the varying overall treatment times, a time factor correction was applied to the BED [Citation24],
(2)
where, T represented the treatment time (days), Tk the time delay time (days) before the start of tumour repopulation, Tp, the cell doubling time and α, the linear parameter as 0.35 Gy−1[Citation24]. The α/β, Tk and Tp for bladder tumours were taken as 15 Gy, 28 days and 5 days respectively as estimated for bladder tumours treated with RT alone [Citation25].
As the patients had received a different dose/fraction for the initial pelvic RT and the boost phases, the BED for each phase was computed separately. The total BED was calculated by deducting the time factor correction, loge2 (T − Tk)/αTp as follows:
(3)
where, OTT represents the overall treatment time (in days) for the entire RT treatment.
With HT at 41 °C for 1 h, it has been reported that the β-component of the cell kill of the L-Q model could increase substantially without any appreciable change in the α-component values. For SiHa tumour cell lines, β-component has been shown to increase more than four-fold (from a mean of 0.02 to 0.09 Gy−2), resulting in a reduction in the mean α/β value from 13.8 Gy−1 to 3.3 Gy−1 with HT [Citation21]. At 43 °C there was also a rise in both the α (from 0.33 to 0.76 Gy−1) and β-components, resulting in a drop in α/β value from 13.8 Gy−1 to 8.7 Gy−1.
It may be noted that SiHa tumour cell lines are derived from human cervical cancers. However, their mean α/β of 13.8 Gy−1 is similar to that of 15 Gy−1 for bladder cancers as estimated by Maciejewski and Majeswski [Citation25]. A similar drop in α/β at 41 °C was also observed for RKO cell lines (derived from human colon cancer) and SW-1573 cell lines (derived from human lung cancer) [Citation21]. We have not come across any other studies that have reported the variation in α/β values at 41 °C in bladder cell lines. The mean bladder temperature in our patients was 41.8 °C for 1 h, and both bladder and cervical cancers have nearly similar α/β at normal temperatures. Thus, a reasonable approximation of reduction of α/β to around 3 Gy−1 with HT, predominantly attributable to an increase in the β-component of the L-Q model could be expected also for the bladder tumours at 41 °C. This could lead to an alteration in the conventional BED values calculated using α/β of 15 Gy−1. We have termed the thermal modulation of conventional BED as the ‘thermo-radiobiological effective dose’ (TBED). Thus, the TBED was recalculated taking the α/β as 3, keeping values of all other variables −Tk, Tp and α, the same.
Chemotherapy
HTCTRT patients were treated with cisplatin (40 mg/m2) weekly for six cycles with standard antiemetic and hydration accordingly to institutional protocol. Patients with a creatinine clearance of <60 mL/min were treated with carboplatin (AUC 2) weekly for a minimum of six cycles. The maximum number of chemotherapy cycles permitted was seven. All patients were monitored for haematological, nephrological, neurological, cardiovascular and other related adverse effects. Supportive treatment and chemotherapy dose modifications were instituted whenever indicated.
Hyperthermia
Deep hyperthermia was delivered using BSD-2000 with a Sigma-60 or Sigma-Eye phased array applicator (Pyrexar Medical, formerly BSD Medical, Salt Lake City, UT, USA) in accordance with the European Society of Hyperthermic Oncology (ESHO) quality guidelines [Citation26,Citation27]. A computed tomography scan was carried out in the HT treatment position with full bladder and the treatment volume was chosen based on the GTV drawn on RT treatment plans. HT consisted of 30 min preheating followed by 60 min of HT treatment. Temperature readings were taken every 10 s in the bladder, rectum, groin, gluteal fold and vagina (in women). Temperature mapping was carried out along the length of the catheter (typically 16 cm long) at every 5 to 10 min. Systemic body temperature, pulse rate, oxygen saturation and blood pressure were continuously monitored during HT. The power settings were set not to exceed a temperature of 43 °C in the bladder, rectum or vagina and were also adjusted according to the patient’s tolerance of HT during the treatment. HT was delivered weekly and the entire treatment lasted for around 90 min. RT was delivered within 15 to 20 min of completion of HT.
Response evaluation and toxicity scoring
Patients were monitored for acute and late morbidities both during and after the completion of treatment. These were mainly skin and soft tissue, urological, gastrointestinal and haematological adverse effects and were recorded as per the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 [Citation28].
Patients were followed up at 6 weeks following the completion of treatment and then every 3 months for the first 2 years and then 6 months thereafter. Cystoscopic biopsies were performed at each follow-up. Local tumour control in the bladder was evaluated by urine cytology and cystoscopy with biopsies and scored as per the RECIST criteria version 1.1 [Citation29]. A tumour was considered to be ‘locally controlled’ only if there were no evidence of any demonstrable tumour on cystoscopy and the biopsies were negative for any malignant lesions. Patients with persistent tumour or carcinoma in situ were considered to have treatment failure.
Regarding the bladder functions following treatment, patient assessments were carried out as ‘patient reported outcomes’ focusing on the symptoms of urinary incontinence, urgency, day time frequency, nocturia, urinary stream and any feeling of incomplete emptying. Patients were specifically asked if they felt satisfied with their bladder functions. Those who did not report any untoward problems in any of the above symptoms and expressed satisfaction with their bladder functions were considered satisfied.
Statistical evaluation
Demographic parameters for patients in the HTRT and HTCTRT groups were compared using chi-square (categorical data) or t-test (continuous data). For survival estimates, the duration of survival was computed from the day of registration till the last follow-up date or death. Bladder preservation was considered as an intact and functional bladder. Local disease-free survival (LDFS) was based on the cystoscopic biopsy, with patients having a negative histopathological report considered as censored. Patients who died due to causes unrelated to their bladder cancers were considered censored for cause-specific survival while deaths due to any cause were counted as events for overall survival. Univariate analysis using Kaplan-Meier and the log-rank test was carried out for the survival end points. A multivariate analysis was performed using the Cox proportional hazard regression model using both demographic and treatment-related variables. Categorical variables were identified and the model was run using a forward conditional method. All statistical tests were two-tailed and p-values less than 0.05 were considered significant. Computations were carried out using SPSS version 21.0 (IBM, Armonk, NY).
Results
Patient demography and treatment offered
All patients were above 65 years of age and not medically fit for radical surgical procedures. The median age was 81 years. Those in the HTRT group had a higher mean age of 82.4 compared to 77.2 years in the HTCTRT (p = 0.06, ). Thus, patients considered for HTRT were not fit to receive concurrent CT. All patients had histopathologically proven urothelial carcinoma and there were no significant differences in sex, Karnofsky performance status, tumour stage, nodal stage, metastatic stage, tumour grade and number of lesions between the two groups (). One patient, aged 77, with a T1 tumour was treated in the HTRT group as he had a grade III tumour and was considered as high risk.
Table 1. Patient and treatment characteristics. Numbers in parenthesis indicate the minimum and maximum values.
Five of the eight patients treated with HTRT had complete resection by TUR-BT. In HTCTRT group, nine of the 12 patients had complete tumour resection. Thus, three patients in each group had macroscopic residual tumour at the start of treatment ().
The mean bladder temperature attained was 41.8 °C. The overall bladder temperature ranged from a minimum of 39 °C to a maximum of 43.7 °C during the entire HT treatments delivered on a weekly basis. There were no significant differences in the temperature profiles in the two groups (). The temperature measured at other sites including rectum, groin, gluteal fold and vagina (in women) were within acceptable limits and comparable in both groups (data not shown).
The mean RT dose received by HTCTRT patients was higher (p < 0.001, ) and was delivered over a longer overall treatment time (p < 0.001). Consequently, patients with HTCTRT also received a higher average number of hyperthermia sessions (5.7 for HTCTRT versus 4.6 for HTRT, p = 0.002). Five to seven cycles of CT (mean 6), usually as carboplatin (n = 10), were administered to the HTCTRT group. Cisplatin was given to two patients with adequate renal functions.
Biological and thermo-radiobiological effective doses
Patients with HTCTRT had a significantly higher OTT, which resulted in a greater time factor correction of nearly four-fold (p < 0.001, ). Although the total BED without time factor correction was significantly higher for HTCTRT patients (p = 0.002), this was nullified by the significantly prolonged OTT. The mean BED with time-factor correction for both groups was therefore similar at 57.8 Gy15 (p not significant). However, the TBED with time-factor correction estimated with an α/β of 3 Gy, showed a significantly higher TBED for HTRT group compared to HTCTRT (p = 0.018, ).
Table 2. Biological effective dose (BED) and thermo-radiobiological effective dose (TBED) for patients treated with thermoradiotherapy (HTRT) and thermochemoradiotherapy (HTCTRT).
Acute and late morbidities
Treatment offered was generally well tolerated by all patients. One HTCTRT patient developed grade III acute skin toxicity which later progressed to skin ulceration and was scored as a grade III late skin toxicity, while another experienced grade III gastrointestinal morbidity. Both patients were treated with HTCTRT and had recovered with conservative treatment. In addition, one patient of HTCTRT had grade III nephrotoxicity in the form of pyelonephritis after the first cycle of cisplatin. The patient then continued on CT with weekly carboplatin. No grade III/IV haematological toxicities were noted in these patients. None of the HTRT patients had any grade III/IV acute or late morbidities.
Bladder preservation
The bladder was preserved and functional in all HTRT patients while two HTCTRT patients underwent cystectomy due to tumour recurrence. Thus, the 3-year bladder preservation rate for all patients was 86.6% (HTRT 100%, HTCTRT 81.5%, p not significant, ). In total 18 of 20 patients who achieved bladder preservation were satisfied with their bladder function and did not report any problems.
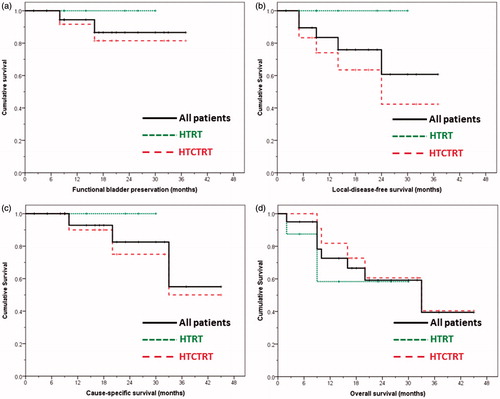
Figure 1. Survival curves for all (n = 20), thermoradiotherapy (HTRT) and thermochemoradiotherapy (HTCTRT) patients for (a) bladder preservation (HTRT versus HTCTRT, log rank p not significant), (b) local disease-free survival (HTRT versus HTCTRT, log rank p = 0.076), (c) cause-specific survival (HTRT versus HTCTRT, log rank p not significant), and (d) overall survival (HTRT versus HTCTRT, log rank p not significant).
Treatment response and survival outcomes
All 20 patients achieved a pathological complete response at 6 weeks following the completion of treatment as evident on cystoscopy and biopsy. During the follow-up (median 17.5 months, range 2–45 months), none of the patients of HTRT had tumour recurrence, while 5/12 HTCTRT recurred locally; 11/20 patients (HTRT 5; HTCTRT 6) were alive and had no evidence of disease at the last follow-up, while 5/20 had died without any disease in the bladder (HTRT 3; HTCTRT 2). In the HTCTRT group, three patients died of disease and one was lost to follow-up as he returned to his native country.
The 3-year LDFS for all patients was 60.7% (HTRT 100%; CTRT 42.3%, p = 0.07, ). As five patients died from a cause unrelated to their primary disease, cause-specific survival was estimated. At 3 years, this was 55% (HTRT 100%; HTCTRT 50%, p not significant, ). The 3-year overall survival for all patients was 39.5% (HTRT 58.3%; HTCTRT 40.4%, p not significant, ).
Multivariate analysis was performed for all the above survival end points. For LDFS, OTT was the only predictive parameter (exp β 1.27, 95% CI 1.03–1.57, p = 0.026; model χ2 = 4.69, p = 0.03). No other variables were found to be of significance for the other survival estimates.
Discussion
Radical cystectomy has often been considered as the ‘gold standard’ in the management of MIBC [Citation30]. However, this may be associated with considerable morbidity and mortality in elderly patients. A recent multicentre review conducted on the role of cystectomy in 111 elderly patients (median age 82.2 years) across Europe and Australia, reported early and late complications to the tune of 50.4% and 32% respectively [Citation31]; 7.2% of the patients died in the immediate post-operative period. At a median follow-up of 18 months, 38.8% of the patients developed tumour progression. The authors concluded that radical cystectomy is an aggressive surgical procedure with a significant complication rate and a careful selection is mandatory to balance the benefit against the risks in elderly patients. Similar concerns have also been voiced by other authors [Citation32–34].
Bladder conservation approaches for MIBC using CT and RT has been accepted as an effective alternative to radical cystectomy, especially in patients unfit for surgery [Citation3,Citation4,Citation8,Citation35,Citation36]. However, the associated toxicities with CTRT in these schedules need careful consideration in elderly patients with existing co-morbidities. It is therefore compelling to explore hyperthermia, a potent radio- and chemosensitiser and without any significant morbidity in patients of MIBC treated without surgery.
Attempts to incorporate HT into therapeutic strategies for MIBC have been encouraging. Kakehi et al. [Citation18] were perhaps the first to report a series of 24 patients of bladder cancers (all stages) treated with either HTRT (n = 16) or HTCT (n = 8). Two patients in each group were reported to have achieved a complete response (CR). The RT dose was 40 Gy delivered at 4 Gy/fraction, twice weekly with Adriamycin, 5-fluorouracil and cisplatin chemotherapy. Matsui et al. [Citation19] reported a triple modality treatment in all stages of bladder cancers using RT (40 Gy/4 weeks) and daily hyperthermic irrigation of the bladder with bleomycin following RT. Of the 56 patients, 25 achieved CR. In stages T2 and T3, they observed a 0% CR with RT alone and 39.4% with the triple modality. Masunaga et al. [Citation16] treated 49 patients with stages T1-4N0M0 bladder cancers to either preoperative RT (24 Gy at 4 Gy/fraction for 2 weeks, n = 21) or the same preoperative RT with HT (n = 28). They observed a 47.6% tumour down-staging with preoperative RT alone compared to 57.1% with combined HTRT with no significant toxicities.
The prospective randomised multicentre trial from the Dutch Deep Hyperthermia Group included patients with MIBC treated with HTRT (n = 52) versus RT alone (n = 49) [Citation17]. A total dose of 66–70 Gy was delivered at 2 Gy/fraction, while HT was prescribed weekly after RT for a total of five treatments. HTRT achieved a CR of 73%, compared to 51% with RT alone (p = 0.01). However, the 3-year overall survival for HTRT was 28% versus 22% with RT alone (p not significant). It was concluded by the authors that RT with or without HT, might not sterilise all the clonogenic cells although they could kill enough to establish a CR.
Wittlinger et al. [Citation20] reported the use of HTCTRT following TUR-BT in 45 patients with high-risk T1 (n = 26) and T2 (n = 19) bladder tumours. Following pelvic RT to 50.4 Gy, a boost of 5.4–9.0 Gy was applied with weekly HT and two cycles of concurrent cisplatin and 5-FU. The median age of the patients was 67 years (range 38–82). In total, 96% achieved CR, while grade III/IV acute and late toxicities were reported in 29% and 24% patients respectively. The 3-year local recurrence-free survival, overall survival, disease-specific survival, metastasis-free survival and bladder preservation rate were 85%, 80%, 88%, 89% and 96% respectively.
The population of patients in the present audit were elderly (median age 81 years) and therefore additional measures had to be taken to offer them a tolerable treatment modality. Risk factors were carefully reviewed to allocate patients to either HTRT or HTCTRT. Furthermore, the RT for HTCTRT patients was relatively prolonged (mean OTT of 43.9 days compared to 30.8 days in HTRT (p < 0.001). This was a consequence of the weekly CT that was planned for HTCTRT patients. It may be noted that despite a higher mean RT dose in HTCTRT group as compared with the HTRT group (57.1 versus 51.0 Gy, p < 0.001), the long-term local disease control was not improved by the addition of CT (long-term disease control 8/8 versus 7/12 in HTRT versus HTCTRT). This led us to evaluate the radiobiological and the thermal modulation of the BED in this patient cohort.
As detailed in , the longer OTT resulted in a significantly higher time factor correction in the HTCTRT group (p < 0.001). This neutralised the higher BED attained with a higher RT dose in the HTCTRT patients. HT at the clinically applied temperatures of 41 °C for 1 h can significantly reduce the β component of the L-Q model [Citation21] resulting in a lower α/β and thus suggesting a benefit when combined with hypofractionated RT. The median dose/fraction in the HTRT group was 2.5 Gy compared to 1.8 Gy in the HTCTRT patients. This resulted in a significantly higher TBED in HTRT patients compared to HTCTRT (HTRT versus HTCTRT: 91.0 ± 4.4 versus 85.8 ± 4.3 Gy3, p = 0.018, ), even though the BED (with time factor corrections) between the two groups were similar.
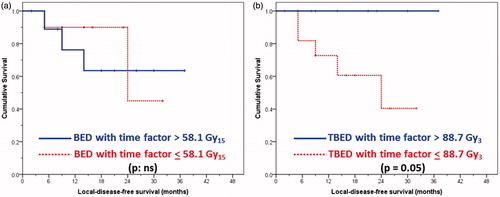
For elderly patients, the associated co-morbidities could influence overall survival. Thus, LDFS needs a closer exploration with respect to the treatment offered in terms of the effective doses delivered with HTRT. Taking median cut-off values with a time factor correction for total BED (as 58.1 Gy15) and TBED (as 88.7 Gy3), it was observed that the LDFS was not influenced by BED (p not significant; ) but was by TBED (p = 0.05, ). Patients with TBED higher than 88.7 Gy3 had a significantly better LDFS; in fact none of these patients had a local disease failure. This provides evidence that the TBED in contrast to conventional BED plays a key role in therapeutic outcomes with HTRT.
Figure 2. Local disease-free survival curves for all 20 patients grouped as (a) patients with biological effective dose (BED) with time factor correction: <58.1 versus >58.1 Gy15 (log rank p not significant), and (b) patients with thermo-radiobiological effective dose (TBED) with time factor correction (see text for details): <88.7 versus >88.7 Gy3 (log rank p = 0.05).
The present audit is limited by the small sample size of elderly patients that we could treat in a span of more than 4 years by HTRT or HTCTRT. However, in-depth analyses of the outcomes of these cases are in accordance with the thermo-radiobiological implications as has been observed in vitro. Further, it also hints towards a possible benefit of hypofractionated RT when used with HT as has been demonstrated in other conditions such as recurrent breast cancer at 4 Gy/fractions [Citation37,Citation38].
However, the advantage of adding CT to HTRT in elderly patients of MIBC still remains open. Most of the patients in the present HTCTRT cohort had received carboplatin, which may not be the most appropriate CT agent [Citation39]. Alternative chemotherapeutic agents such as gemcitabine, mitomycin C and 5-FU may need consideration for patients unfit for cisplatin. The advantage of adding CT to HTRT and the optimal integration of these three modalities in elderly patients needs further evaluation through prospective studies.
Thus, in view of the outcomes from this analysis, HT along with a shorter overall treatment with a relatively higher dose per fraction could be contemplated for elderly patients who are not fit for CTRT. Future randomised trials should also take into consideration the impact of HT on the BED. Treatment protocols designed with an optimal HTRT dose–fractionation schedule with a relatively higher dose/fraction delivered in a shorter OTT should be formulated and compared with standard CTRT or HTCTRT. These schedules should also be commensurate with avoiding or minimising late toxicity to the normal pelvic organs. Such a tailored HTRT regimen could perhaps achieve better clinical outcome without the need for additional CT in elderly patients with MIBC. This could help avoid radical surgical procedures and improve quality of life by preserving a functional bladder in these patients.
Acknowledgements
We thank Susanne Rogers for reviewing the manuscript.
Declaration of interest
This study has been supported by the partial grant from Research Council, Kantonnspital Aarau (Forschungrsat KSA). The authors alone are responsible for the content and writing of the paper.
References
- Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, et al. GLOBOCAN 2012 v1.0, The Globocan Project. Cancer incidence and mortality worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer; 2013. Available from http://globocan.iarc.fr. (accessed 17 November 2015)
- Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Canc Netw 2013;11:446–75
- Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol 2013;63:45–57
- Witjes JA, Comperat E, Cowan NC, De Santis M, Gakis G, Lebret T, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol 2014;65:778–92
- Martini T, Mayr R, Wehrberger C, Dechet C, Lodde M, Palermo S, et al. Comparison of radical cystectomy with conservative treatment in geriatric (≥80) patients with muscle-invasive bladder cancer. Int Braz J Urol 2013;39:622–30
- Koga F, Kihara K. Selective bladder preservation with curative intent for muscle-invasive bladder cancer: A contemporary review. Int J Urol 2012;19:388–401
- Yoo S, You D, Jeong IG, Hong JH, Ahn H, Kim CS. Does radical cystectomy improve overall survival in octogenarians with muscle-invasive bladder cancer? Korean J Urol 2011;52:446–51
- Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9
- James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med 2012;366:1477–88
- Huddart RA, Hall E, Hussain SA, Jenkins P, Rawlings C, Tremlett J, et al. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: Results of the BC2001 trial (CRUK/01/004). Int J Radiat Oncol Biol Phys 2013;87:261–9
- Smith ZL, Christodouleas JP, Keefe SM, Malkowicz SB, Guzzo TJ. Bladder preservation in the treatment of muscle-invasive bladder cancer (MIBC): A review of the literature and a practical approach to therapy. BJU Int 2013;112:13–25
- Ploussard G, Daneshmand S, Efstathiou JA, Herr HW, James ND, Rodel CM, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: A systematic review. Eur Urol 2014;66:120–37
- Datta NR, Ordonez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, et al. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat Rev 2015;41:742–53
- Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int 2011;107:912–18
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: A systematic review. Eur Urol 2011;60:81–93
- Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, et al. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyperthermia 1994;10:31–40
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000;355(9210):1119–25
- Kakehi M, Ueda K, Mukojima T, Hiraoka M, Seto O, Akanuma A, et al. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int J Hyperthermia 1990;6:719–40
- Matsui K, Takebayashi S, Watai K, Kakehi M, Kubota Y, Yao M, et al. Combination radiotherapy of urinary bladder carcinoma with chemohyperthermia. Int J Hyperthermia 1991;7:19–26
- Wittlinger M, Rodel CM, Weiss C, Krause SF, Kuhn R, Fietkau R, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: Transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol 2009;93:358–63
- Franken NA, Oei AL, Kok HP, Rodermond HM, Sminia P, Crezee J, et al. Cell survival and radiosensitisation: Modulation of the linear and quadratic parameters of the LQ model (Review). Int J Oncol 2013;42:1501–15
- ICRU. Prescribing, Recording, and Reporting Photon Beam Therapy (ICRU Report 50). Bethesda, MD: ICRU, 1993
- ICRU. Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50), ICRU Report 62. Bethesda, MD: ICRU, 1999
- Fowler JF. 21 years of biologically effective dose. Br J Radiol 2010;83:554–68
- Maciejewski B, Majewski S. Dose fractionation and tumour repopulation in radiotherapy for bladder cancer. Radiother Oncol 1991;21:163–70
- Lagendijk JJ, Van Rhoon GC, Hornsleth SN, Wust P, De Leeuw AC, Schneider CJ, et al. ESHO quality assurance guidelines for regional hyperthermia. Int J Hyperthermia 1998;14:125–33
- Bruggmoser G, Bauchowitz S, Canters R, Crezee H, Ehmann M, Gellermann J, et al. Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 2011;187:605–10
- US Department of Health and Human Services NCI. Common Termionology Criteria for Adverse Events (CTCAE) version 4.0. Bethesda, MD: National Institutes of Health, 2010. version 4.03: Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf (accessed 17 November 2015)
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47
- Kiss B, Burkhard FC, Thalmann GN. Open radical cystectomy: Still the gold standard for muscle invasive bladder cancer. World J Urol 2015;Nov 19 [Epub ahead of print]
- Izquierdo L, Peri L, Leon P, Ramirez-Backhaus M, Manning T, Alcaraz A, et al. The role of cystectomy in elderly patients – a multicentre analysis. BJU Int 2015;116(Suppl3):73–9
- Bjornsson O, Gudmundsson EO, Marteinsson VT, Jonsson E. Radical cystectomy in the treatment of bladder cancer in Iceland: A population-based study. Scand J Urol 2015;Sept 21:1–6 [Epub ahead of print]
- Garde H, Ciappara M, Galante I, Fuentes Ferrer M, Gomez A, Blazquez J, et al. Radical cystectomy in octogenarian patients: A difficult decision to take. Urol Int 2015;94:390–3
- Zakaria AS, Santos F, Tanguay S, Kassouf W, Aprikian AG. Radical cystectomy in patients over 80 years old in Quebec: A population-based study of outcomes. J Surg Oncol 2015;111:917–22
- Zagouri F, Peroukidis S, Tzannis K, Kouloulias V, Bamias A. Current clinical practice guidelines on chemotherapy and radiotherapy for the treatment of non-metastatic muscle-invasive urothelial cancer: A systematic review and critical evaluation by the Hellenic Genito-Urinary Cancer Group (HGUCG). Crit Rev Oncol Hematol 2015;93:36–49
- Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur Urol 2012;61:705–11
- Linthorst M, van Geel AN, Baaijens M, Ameziane A, Ghidey W, van Rhoon GC, et al. Re-irradiation and hyperthermia after surgery for recurrent breast cancer. Radiother Oncol 2013;109:188–93
- van der Zee J, De Bruijne M, Mens JW, Ameziane A, Broekmeyer-Reurink MP, Drizdal T, et al. Reirradiation combined with hyperthermia in breast cancer recurrences: Overview of experience in Erasmus MC. Int J Hyperthermia 2010;26:638–48
- National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology, bladder cancer 2015. version 2.2015. Available from http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed 4 December 2015)
