Abstract
Purpose: The aim of this study was to evaluate radiofrequency ablation (RFA) and microwave ablation (MWA) as a viable salvage option for patients with locally recurrent non-small cell lung cancer (NSCLC) after radiotherapy. Materials and methods: This retrospective study was conducted on patients who had received thermal ablation for recurrent NSCLC post-curative radiotherapy. Medical records and follow-up imaging with computed tomography (CT) and PET-CT were analysed to determine time to local progression (TTLP) and overall survival (OS). TTLP was determined according to the modified RECIST criteria. Results: Twelve patients, mean age 71 ± 7 years, received 17 thermal ablation sessions, with RFA performed for four lesions and MWA for 13. Nine tumours were squamous cell cancers (SCC) and eight were adenocarcinomas. Eleven tumours had recurred post-external beam radiation and one post-stereotactic body radiation therapy. Mean tumour size was 34.2 ± 12.8 mm, tumour stages prior to radiotherapy were Ia (2), Ib (3), IIa (4), IIb (1) and III (2). Follow-up period was 19 ± 11 months. Overall median TTLP was 14 months (95% CI: 8, 19), and median OS was 35 months (95% CI: 12, 58). Mean TTLP for tumours <30 mm was 23 months and for tumours >30 mm 14 months (p = 0.20). Recurrence rates reduced from 50% after initial ablation to 20% with a second ablation. Complication rate for pneumothorax requiring intervention was 17%. Conclusion: Both RFA and MWA ablation prolonged local tumour control with minimal morbidity in this study group of recurrent NSCLC after radiotherapy.
Introduction
Lung cancer is currently the leading cause of cancer-related mortality for both men and women worldwide, making it an important global health issue [Citation1]. For early stage non-small cell lung cancer (NSCLC) the standard treatment is surgical resection, which achieves the best long-term local control [Citation2]. However, at least 20% of patients are unable to have surgery due to medical comorbidities [Citation3]. The treatment of choice for non-surgical candidates, or those who refuse surgery with early stage disease, is radiotherapy. However, a Cochrane review of over 2000 medically inoperable patients treated with conventional radiotherapy alone demonstrated relatively low 5-year overall survival rates of 0–42%, and relatively high recurrence rates ranging from 6–70% [Citation4]. Newer radiotherapy techniques such as stereotactic body radiotherapy (SBRT) and intensity modulated radiotherapy (IMRT) have improved local recurrence rates, particularly for stage I NSCLC [Citation5,Citation6]; however, recurrence rates are still high, being around 27% at 3 years for SBRT [Citation7] and 50% at 2 years for IMRT [Citation8] for stage I NSCLC. Once local recurrence occurs within the radiation field, further irradiation is usually not a valid retreatment option. The objective of this study is to retrospectively assess time to local progression (TTLP) and overall survival (OS) for percutaneous computed tomography (CT)-guided thermal ablation including both radiofrequency ablation (RFA) and microwave ablation (MWA) performed at a tertiary cancer hospital on patients with local tumour recurrence after radiotherapy. This was conducted with the aim of evaluating thermal ablation as a viable salvage therapy for patients with recurrent NSCLC after radiotherapy.
Materials and methods
Patients were identified using a radiology thermal ablation database at a large tertiary hospital. Data was collected retrospectively through systematic review of patient charts and imaging. This study was undertaken under audit provisions in the national statement.
Subject selection
Inclusion criteria were consecutive patients with biopsy-confirmed NSCLC who underwent percutaneous CT-guided thermal ablation between 2008 and 2015, and had received prior radical radiotherapy. At the time of presentation for thermal ablation, these patients had local tumour recurrence and were not deemed suitable for further radiation or surgery according to consensus from the pulmonary malignancy multidisciplinary team (MDT) meeting.
Exclusion criteria
Patients were excluded from analysis if radiotherapy did not precede thermal ablation or the new target was outside the irradiated lung field.
Thermal ablation
Prior to the procedure, blood tests including full blood count and coagulation profile were performed. No peri-interventional prophylactic antibiotics were administered. Both RFA and MWA thermal ablation modalities were used (with MWA having replaced RFA by mid-2010). CT guidance was used for device placement in all patients. The choice of conscious sedation or general anaesthesia was left to the anaesthetic team in charge at the time of procedure. All ablations were performed by a single interventional radiologist with greater than 10 years’ experience in lung ablation. Parameters such as type of applicator, number of overlapping ablations, ablation duration and amount of energy delivered were based on individual tumour size, location and lesion characteristics. Limited non-contrast enhanced CT imaging was performed 24 h post-procedure in all MWA cases, as the imaging immediately after MWA tends to underestimate the final MW ablation zone. Technical success was defined as the ability to conclude the ablation procedure without immediate issues prompting the abortion of the intervention. An additional method to confirm technical ablation success was by assessing a circumferential rim of ground glass opacity (GGO) surrounding the tumour, although this was often precluded by pre-existing radiation-induced consolidation, atelectasis, and/or traction at the site. Patients were managed in the post-anaesthesia care unit immediately after the procedure and transferred to a 23-h ward once fully awake and stable.
Data collection and evaluation
Clinical outcomes were determined on the basis of review of medical records, follow-up imaging, histopathology of any biopsy-proven residual or recurrent disease, and survival status from the state oncology database. Patient data including age, gender, histology, stage, and previous radiotherapy, chemotherapy or surgery was collected from medical charts. Serial CT images and 18fluorodeoxyglucose positron emission tomography (18F-FDG PET) studies were reviewed to determine TTLP.
Modified Response Evaluation Criteria In Solid Tumors (RECIST) were used to categorise response to ablation therapy as: complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).[Citation5,Citation9] To apply the modified RECIST criteria, serial CT imaging and PET-CT studies were compared to baseline CT imaging and PET-CT studies respectively. Tumours were ablated with an intended circumferential safety margin around the tumour, causing congestion, haemorrhage and an inflammatory response, resulting in an unavoidable increase in ablation volume [Citation5] identified on follow-up CT images. Therefore, baseline was taken as the first post-ablation CT scan, usually performed after 24 h, rather than pre-ablation scans. TTLP was defined as the time interval between ablation and PD. According to the modified RECIST Criteria, PD was defined as an increase in size, change in tumour morphology on CT imaging, or increased uptake on FDG-PET studies [Citation5,Citation9]. OS was defined as the time interval between first ablation and death recorded on the state oncology database.
Statistical analysis
The primary clinical outcomes of TTLP and OS were analysed using the Kaplan-Meier method, which generated disease recurrence and survival curves. TTLP was analysed per lesion, whereas OS was analysed per patient. Patients who were alive were censored. The clinical outcomes (TTLP and OS) were compared for nodule size (<30 mm and >30 mm). This comparison was analysed through a log rank test. Statistical significance was considered at a value of p < 0.05. All statistical analysis was performed using SPSS Statistics version 22 (IBM, Armonk, NY).
Results
Exclusions
From the hospital’s thermal ablation database, 17 patients were identified as having received radiotherapy and thermal ablation. Five patients were excluded, two of which because the radiotherapy target was outside lung fields, and the other three because radiotherapy had not been delivered to the new target lesion prior to thermal ablation.
Subject and tumor characteristics
This study analysed 17 tumour ablation sessions in 12 patients who each had histologically proven solitary NSCLC and had previously received radical radiotherapy (external beam radiotherapy in 11 patients, SBRT in one patient) to those tumours. All 12 patients received an initial thermal ablation session for their recurrent tumour in the previously irradiated field. Five patients required re-ablation for local recurrence after initial thermal ablation, independent of technical failure. The subject and tumour characteristics of the patients are summarised in .
Table 1. Subject, tumour and imaging characteristics, and outcomes of patients receiving thermal ablation for non-small cell lung cancer local recurrence after radiotherapy.
Thermal ablation characteristics
RFA was performed on two patients who required an initial ablation and re-ablation (four RFA cases in total). In one patient RFA was performed using a Valleylab device (Cool-tip Valleylab, Covidien, Boulder, CO, USA), initially using a 2-cm active tip followed by a 3-cm active tip at re-ablation. The other patient had their ablation and re-ablation performed using an AngioDynamics device with deployable tines (Starburst-XL, AngioDynamics, Latham, NY). MWA was performed in 13 tumours. 12 cases were performed using an AngioDynamics device (Acculis MTA, AngioDynamics) with a 2.45 GHz generator and 16-mm active tip. The remaining case was performed using a 915-MHz Evident MWA System (Covidien) with a 20-mm active tip.
Imaging characteristics
Generally, all patients were followed up at 3, 6, 12 months, then half-yearly CT scans, and a 3–6 month PET/CT scan. All patients receiving MWA were followed up 24 h after ablation with a non-contrast enhanced CT scan. Total follow-up time per patient was 19 ± 11 months. Follow-up times per lesion are summarised in .
Table 2. Treatment characteristics of patients receiving thermal ablation for NSCLC local recurrence after radiotherapy.
Treatment characteristics
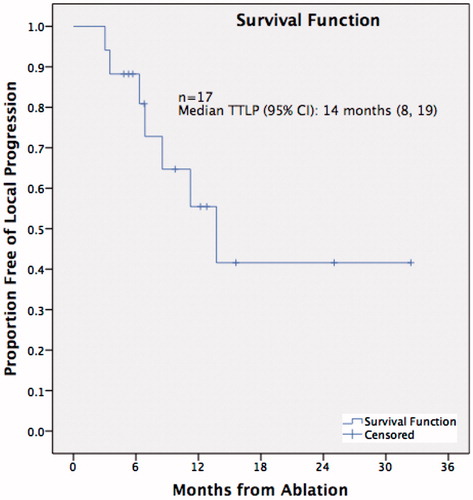
Technical success was achieved in 100% (n = 17) of thermal ablation sessions. Immediate, peri-procedural, and delayed complications were recorded on a per-treatment basis and were classified in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) of the US National Cancer Institute [Citation10] There were no intra-procedural or 30-day post-procedural deaths. The ablations were complicated by five pneumothoraces (29%), with two (12%) requiring a chest drain insertion. Local tumour recurrence occurred in 6/12 (50%) of patients, of whom five patients (83%) went on to have a second ablation and one did not, as they died prior to any re-ablation therapy. There was one tumour recurrence after the second ablation (1/5, 20%). Median TTLP following ablation was 14 months (95% CI: 8, 19). Local progression rate at 1 year was 45%. shows the Kaplan-Meier curve of TTLP. In the cohort, seven (58%) patients died from causes unrelated to the treated lung cancer with a mean follow-up of 19 months. Median survival was 35 months (95% CI: 12, 58). The treatment characteristics are summarised in .
Tumour size and time to local progression
Tumours <30 mm had a longer TTLP of 23 months (95% CI: 13, 33), compared to tumours >30 mm having a TTLP of 14 months (95% CI: 7, 20), although this did not reach statistical significance (p = 0.20). Mean survival was slightly longer for tumours <30 mm which was 38 months (95% CI: 22, 54), compared to tumours >30 mm which was 35 months (95% CI: 16, 54). This also did not reach statistical significance (p = 0.20).
Discussion
There are limited treatment options available for patients with recurrent lung tumours after radiotherapy. Although thermal ablation has been used for the treatment of lung tumours for well over a decade, literature on its role as salvage therapy in locally recurring lung cancer is limited. To our knowledge there are only two published studies evaluating the clinical outcomes of thermal ablation for recurrent NSCLC arising from previously irradiated lung fields. One study of 33 patients by Schoellnast et al. [Citation11] evaluated RFA as salvage therapy after radiotherapy, chemotherapy and surgery collectively. The other study by Leung et al. [Citation12] of 20 patients evaluated outcomes in RFA and MWA for previously irradiated patients. Median TTLP between our study and Schoellnast et al. [Citation11] matched at 14 months, while the TTLP of 3 months reported by Leung et al. [Citation12] was considerably shorter. This is likely attributable to their larger mean tumour size of 45 mm, compared to our mean tumour size of 34 mm and Schoellnast’s of 28 mm. There is strong evidence demonstrating a negative correlation between tumour size and complete treatment response [Citation13,Citation14]. The TTLP in our study was also comparable to that reported for primary NSCLC treated with RFA [Citation15,Citation16]. Comparing outcomes between thermal ablation after radiation therapy and as primary treatment of NSCLC is challenging; post-radiation fibrosis and atelectatic changes often obscure the boundaries of recurrent tumours, which impairs the accurate device positioning and makes complete treatment difficult. Additionally, applying the modified RECIST criteria for follow-up in the given patient population is difficult due to limitations of response assessibility in previously irradiated lungs. Some patients in our study showed complete clinical treatment response with adequate follow-up times, but were deemed partial response (PR) or stable disease (SD) under the modified RECIST criteria. Despite this, the TTLP in our study (14 months) was within the range of 9–14 months reported in studies on RFA of primary NSCLC [Citation15,Citation16]. Similar tumour sizes were treated, with mean tumour size of 24 mm for Hiraki et al. [Citation15], 26 mm for Pennathur et al. [Citation16], and 27 mm in our study. Therefore, the similar local progression outcomes between thermal ablation post-radiation therapy and as primary treatment suggests that thermal ablation could be a viable and successful salvage option, despite the associated difficulties in treating previously irradiated fields.
Our more favourable results of a longer OS of 35 months and lower mortality of 58% compared to Schoellnast et al. (OS of 21 months and mortality of 64%) with a similar follow-up period and similar patient characteristics, is possibly due to the majority of our cohort being treated with MWA (only two patients received RFA) and being re-ablated (42% in our study compared to 9% with Schoellnast) thus likely providing more aggressive local control. In support of this, repeat RFA was shown to improve local tumour control in a study of 797 lung tumours [Citation17]. One of the unique strengths of thermal ablation is its repeatability in the setting of tumour recurrence, with little morbidity and no added toxicity compared to other treatment modalities such as surgery or radiotherapy. demonstrate the benefit of re-ablation for local tumour control following recurrence after one ablation.
Figure 2. A 55-year-old patient with recurrence of right upper lobe (RUL) non-small cell lung cancer (NSCLC) after 60 Gy of radical external beam radiation. (a) Axial CT lung window shows lobulated RUL mass. (b) Central position of microwave antenna within the tumour. (c) Axial CT scan 24 h after MWA shows surrounding ground-glass opacity (GGO) around tumour (arrows); medial lack of GGO at level of dashed arrows. (d) Volumetric comparison between 3 months post-ablation scan and 6 months post-ablation scan showing reduction in size by 30%, qualifying as partial response under RECIST criteria.
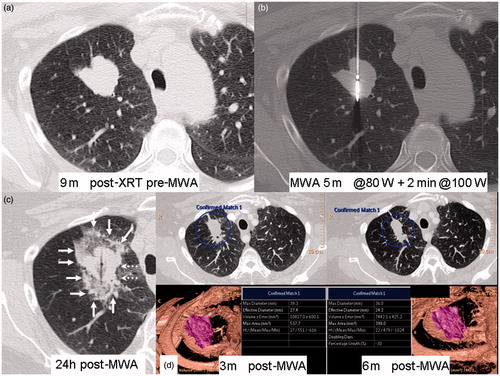
Figure 3. A 55-year-old patient with recurrence of right right upper lobe (RUL) non-small cell lung cancer (NSCLC) after 60 Gy of radical external beam radiation, treated with MWA. Residual avidity on FDG-PET scan 6 months post-ablation (see ). (a) CT-guided core biopsy RUL lesion. (b) CT-guided microwave re-ablation 12 months after initial ablation (c) Axial CT scan 24 h after re-ablation shows large circumferential area of GGO and partial atelectasis. (d) CT scan 6 months after re-ablation shows cavitating non-enhancing ablation site.
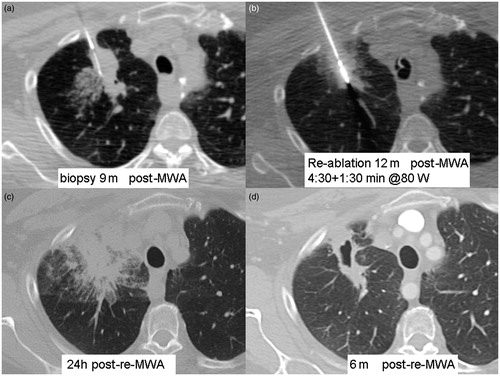
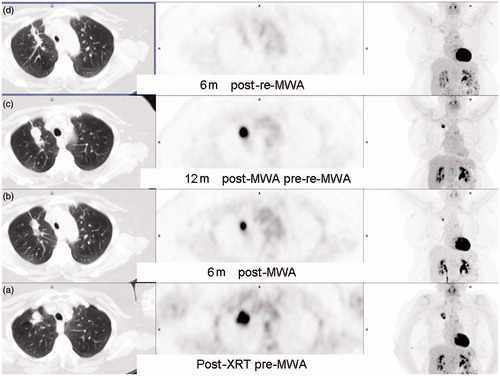
Figure 4. A 56-year-old patient with right upper lobe (RUL) non-small cell lung cancer (NSCLC) with FDG-PET scan series. (a) After 30 cycles x 2 Gy of radical external beam radiotherapy. (b) Six months after MWA shows residual inferior FDG uptake, best appreciated on the coronal MIP. (c) welve months after MWA shows enlarging RUL mass with increasing FDG uptake. (d) Six months after re-MWA shows shrinking focus with no residual FDG uptake.
The complication rate in our study was also similar to the rates reported in the literature. Pneumothorax occurred in 29% of our patients, with reported rates ranging from 13–52% in thermal ablation as primary treatment for NSCLC [Citation13,Citation18,Citation19]. Our 12% chest tube requirement is within the range of 10–20% reported in other studies [Citation13,Citation20]. The uncomplicated nature of small asymptomatic pneumothoraxes and the usually short-term chest tube insertion qualifies the severity of the complication as CTCAE 1 (no chest tube) or CTCAE 2. Pneumothoraces requiring intervention occurred more frequently in larger lesions close to the chest wall, which required overlapping ablations and longer ablation durations. While the acute complications of thermal ablation outweigh those of SBRT, because of the invasive nature of thermal ablation this reverses in the longer term, as thermal ablation is associated with far fewer chronic complications such as chest wall pain beyond the acute phase, chronic cough and rib fractures. As such, quality of life is a priority in the setting of salvage therapy, and small asymptomatic pneumothoraces are unlikely to negatively affect the quality of life of the patient. There were no other significant post-ablation complications in our study.
MWA has recently been emerging as a new modality to treat lung tumours, both primary and metastatic [Citation21–28]. MWA has several theoretical advantages over RFA, mostly because of different mechanisms used to generate frictional heat. MWA, employing much higher frequencies (915 MHz and 2.45 GHz vs 460–500 kHz for RFA) uses direct heating, rather than resistive heating in RFA, enabling it to reach higher temperatures and to generate a much larger and more homogenous zone of thermal coagulation in a shorter time and significantly less heat sink effect [Citation29,Citation30]. Additionally it allows for multiple applicators to be used simultaneously, which improves ablative volume and margins [Citation31].
The major limitation of this study is its small sample size and its retrospective design. SBRT is considered the gold standard for non-surgical treatment of node-negative small NSCLC [Citation32,Citation33]. Unfortunately, at our institution we are unable to offer SBRT to our lung cancer patients on a routine basis, which precluded a prospective randomised trial. The heterogeneity in our small patient population with varying stages of disease and co-morbidities may have skewed clinical outcomes. Some tumours were treated with chemotherapy in conjunction with radiotherapy which may alter the biology of the tumour and ultimately affect the aggressiveness of the treated malignancies. Additionally, treated tumours were a mixture of squamous cell carcinomas and adenocarcinomas, not controlling for tumour biology. Our overall clinical outcomes and complication rates still correlated with similar studies on larger cohorts published in the literature. This provides substantiation to the use of thermal ablation as a salvage therapy, especially considering that these patients have few alternative treatment options. Furthermore, outcomes of thermal ablation in combination with radiotherapy and chemotherapy are promising, with emerging evidence of its superiority over single modality therapy [Citation34,Citation35]. Synergy between radiotherapy and thermal ablation is thought to occur because radiation is most effective against well-oxygenated cells in the tumour periphery and less effective at targeting more hypoxic cells in the centre, whereas thermal ablation targets the core but is less effective in the periphery due to increasing heat sink effects [Citation36,Citation37]. Recently, applicators that simultaneously deliver radiation and thermal ablation therapy have been developed, which potentially offers more effective treatment [Citation38].
Conclusion
Our study suggests that thermal ablation can be a useful salvage option for recurrent NSCLC post-radiotherapy. There is scope for future prospective studies further evaluating the outcomes of thermal ablation therapy and comparing it to SBRT in randomised trials.
Acknowledgements
The authors would like to thank Dr Frank Fiumara, staff specialist in nuclear medicine, for his help with compiling the images and Drs Simon Gray and Lachlan McDowell for their contribution during the early stages of this manuscript.
Declaration of interest
There is no funding to declare in this study and no authors of this study have any conflict of interests to declare. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer, 2013. Available from http://globocan.iarc.fr
- Van Schil PE. Surgery for non-small cell lung cancer. Lung Cancer 2001;34(Suppl2):S127–32
- El-Sherif A, Gooding WE, Santos R, Pettiford B, Ferson PF, Fernando HC, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: A 13-year analysis. Ann Thorac Surg 2006;82:408–15; discussion 15–16
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). Cochrane Database Syst Rev 2001;2:CD002935
- Abbas G, Schuchert MJ, Pennathur A, Gilbert S, Luketich JD. Ablative treatments for lung tumors: Radiofrequency ablation, stereotactic radiosurgery, and microwave ablation. Thorac Surg Clin 2007;17:261–71
- O’Rourke N, Roque IFM, Farre Bernado N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2010;6:CD002140
- Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352–8
- Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: The Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol 2008;87:17–23
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47
- US National Caner Institute. Common terminology criteria for adverse events (CTCAE) version 4.03. US Depatment of Health and Human Services, 2010, revised 2014. Available from http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- Schoellnast H, Deodhar A, Hsu M, Moskowitz C, Nehmeh SA, Thornton RH, et al. Recurrent non-small cell lung cancer: Evaluation of CT-guided radiofrequency ablation as salvage therapy. Acta Radiol 2012;53:893–9
- Leung VA, DiPetrillo TA, Dupuy DE. Image-guided tumor ablation for the treatment of recurrent non-small cell lung cancer within the radiation field. Eur J Radiol 2011;80:e491–9
- Simon CJ, Dupuy DE, DiPetrillo TA, Safran HP, Grieco CA, Ng T, et al. Pulmonary radiofrequency ablation: Long-term safety and efficacy in 153 patients. Radiology 2007;243:268–75
- Choe YH, Kim SR, Lee KS, Lee KY, Park SJ, Jin GY, et al. The use of PTC and RFA as treatment alternatives with low procedural morbidity in non-small cell lung cancer. Eur J Cancer 2009;45:1773–9
- Hiraki T, Gobara H, Iishi T, Sano Y, Iguchi T, Fujiwara H, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: Results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg 2007;134:1306–12
- Pennathur A, Luketich JD, Abbas G, Chen M, Fernando HC, Gooding WE, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2007;134:857–64
- Hiraki T, Mimura H, Gobara H, Sano Y, Fujiwara H, Date H, et al. Repeat radiofrequency ablation for local progression of lung tumors: Does it have a role in local tumor control? J Vasc Intervent Radiol 2008;19:706–11
- Hiraki T, Tajiri N, Mimura H, Yasui K, Gobara H, Mukai T, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: Incidence and risk factors. Radiology 2006;241:275–83
- Lanuti M, Sharma A, Digumarthy SR, Wright CD, Donahue DM, Wain JC, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160–6
- Lencioni R, Crocetti L, Cioni R, Suh R, Glenn D, Regge D, et al. Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621–8
- Acksteiner C, Steinke K. Percutaneous microwave ablation for early-stage non-small cell lung cancer (NSCLC) in the elderly: A promising outlook. J Med Imaging Radiat Oncol 2015;59:82–90
- Carrafiello G, Mangini M, Fontana F, Di Massa A, Ierardi AM, Cotta E, et al. Complications of microwave and radiofrequency lung ablation: Personal experience and review of the literature. Radiologia Medica 2012;117:201–13
- Jahangeer S, Forde P, Soden D, Hinchion J. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev 2013;39:862–71
- Little MW, Chung D, Boardman P, Gleeson FV, Anderson EM. Microwave ablation of pulmonary malignancies using a novel high-energy antenna system. Cardiovasc Intervent Radiol 2013;36:460–5
- Lu Q, Cao W, Huang L, Wan Y, Liu T, Cheng Q, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol 2012;10:80
- Splatt AM, Steinke K. Major complications of high-energy microwave ablation for percutaneous CT-guided treatment of lung malignancies: Single-centre experience after 4 years. J Med Imaging Radiat Oncol 2015;59:609–16
- Steinke K, Liu H. Minimally invasive techniques for medically inoperable stage I non-small cell lung cancer (NSCLC) – image-guided microwave ablation, a promising therapy option. J Med Imaging Radiat Oncol 2014;58:79–80
- Vogl TJ, Naguib NN, Gruber-Rouh T, Koitka K, Lehnert T, Nour-Eldin NE. Microwave ablation therapy: Clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643–51
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics 2005;25:S69–83
- Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: Influence of hepatic vein size on heat-sink effect in a porcine model. J VascI Intervent Radiol 2008;19:1087–92
- Durick NA, Laeseke PF, Broderick LS, Lee FT, Jr, Sampson LA, Frey TM, et al. Microwave ablation with triaxial antennas tuned for lung: Results in an in vivo porcine model. Radiology 2008;247:80–7
- Shirvani SM, Chang JY, Roth JA. Can stereotactic ablative radiotherapy in early stage lung cancers produce comparable success as surgery? Thorac Surg Clin 2013;23:369–81
- Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(Suppl5):e278–313
- Dupuy DE, DiPetrillo T, Gandhi S, Ready N, Ng T, Donat W, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006;129:738–45
- Grieco CA, Simon CJ, Mayo-Smith WW, DiPetrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: Outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Intervent Radiol 2006;17:1117–24
- Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: Exploring mechanisms of interaction. Clin Cancer Res 2003;9:1957–71
- Jain SK, Dupuy DE, Cardarelli GA, Zheng Z, DiPetrillo TA. Percutaneous radiofrequency ablation of pulmonary malignancies: Combined treatment with brachytherapy. Am J Roentgenol 2003;181:711–15
- Kosterev VV, Kramer-Ageev EA, Mazokhin VN, van Rhoon GC, Crezee J. Development of a novel method to enhance the therapeutic effect on tumours by simultaneous action of radiation and heating. Int J Hyperthermia 2015;31:443–52