Abstract
Although many treatment modalities and schedules for non-muscle-invasive bladder cancer (NMIBC) exist, all yet prove to have limitations. Therefore the search for new forms of therapy continues. One of these forms consists of combining intravesical chemotherapy, typically mitomycin C (MMC), with hyperthermia achieved by a microwave-applicator. We aimed to review the current status of intravesical radiofrequency (RF) induced chemohyperthermia (CHT) for NMIBC with regard to efficacy, adverse-events (AEs) and its future perspective. A search for RF-induced CHT in MEDLINE, Embase, Cochrane and ClinicalTrials.gov databases was performed. Relevant conference abstracts were searched for manually. If applicable, experts on the area were consulted. Papers were selected based on abstract and title. A table of newly published clinical trials since 2011 was constructed. No meta-analysis could be performed based on these new papers. Efficacy proved to be better for RF-induced CHT compared to both MMC alone and bacillus Calmette–Guérin (BCG) instillations, with the latter being based on just one abstract of a randomised controlled trial. The AE rate in CHT is higher compared to MMC instillation, but is similar compared to BCG, albeit different in the type of AE. In almost all studies no severe AEs are reported. Although heterogeneity in methodology exists, RF-induced CHT seems promising. However, alternative methods of applying hyperthermia are starting to present their first results, imposing as effective options too. Intravesical RF-induced CHT may become an alternative for BCG instillation, and possibly for cystectomy, although further level 1 evidence is required for both reliable and reproducible data on efficacy and adverse events.
Introduction
Bladder cancer is the most common malignancy of the urinary tract. In the European Union (EU), the age-standardised incidence rate varies from 6 per 100 000 in women to 27 per 100 000 in men [Citation1]. These rates vary per region within the EU. On top of the high incidence, bladder cancer was the eighth most common cause of cancer-specific mortality in 2008.
On presentation, about 75% of patients are diagnosed with non-muscle-invasive bladder cancer (NMIBC). In these patients urothelial cell carcinoma is confined to the mucosa (stage Ta, carcinoma in situ (CIS)) or invades the lamina propria only (stage T1). The remaining patients present with muscle-invasive disease (T ≥ 2) which can be treated with radical cystectomy (RC), radiotherapy or systemic chemotherapy, depending on the presence and extent of regionally or distantly spread disease.
Fortunately, both incidence and mortality are decreasing in some registries, possibly reflecting a decreased prevalence of smoking, less occupational exposure to causative agents, and improved care. As opposed to muscle-invasive bladder cancer (MIBC), NMIBC has a high prevalence due to relatively low progression rates (5-year progression rate of 1–45% (0–30% in the Club Urológico Español de Tratamiento Oncológico (CUETO) risk tables), depending on grade, stage, tumour size, number of tumours, presence of CIS, previous recurrences according to the European Organization for Research and Treatment of Cancer (EORTC) risk tables [Citation2] and additionally sex and age according to the CUETO risk tables [Citation3]) and thus has a long patient survival. However, 5-year recurrence rates can be as high as 78%, necessitating a frequent and long-term follow-up with outpatient clinic visits, cytology tests, and invasive cystoscopies. This imposes a significant burden on the patient and on the healthcare system in both financial and logistic way.
To prevent recurrence and progression from occurring, adjuvant treatment after trans-urethral resection of a bladder tumour (TURBT) is given. According to the EAU guideline on NMIBC, a single post-operative instillation (SPI) with chemotherapy, a 1-year protocol with chemotherapy or immunotherapy with bacillus Calmette–Guérin vaccine (BCG), or instillations with BCG for 3 years should be given for low, intermediate and high risk disease, respectively [Citation1]. However, in the above-mentioned CUETO risk tables for recurrence and progression, these instillation protocols were already taken into account, and still recurrence rates are high. Moreover, a world-wide shortage in BCG has currently evolved. The shortage is even more stringent because of the importance of BCG in patients at high risk for progression to MIBC.
For these reasons, extensive research has been performed to improve therapies in both adjuvant and neo-adjuvant settings to prevent recurrence and progression, reduce the intensity of follow-up, and to maximise bladder preservation rates, thus prolonging the patient's quality of life and survival. A new treatment modality is electromotive drug administration (EMDA) [Citation4], in which iontophoresis is being used for improving tissue penetration by the chemotherapeutic drug. Another strategy is photodynamic therapy (PDT), which uses the interaction between absorbed light and a retained photosensitising agent to destroy tissue, although it is infrequently applied. A final new and promising strategy is hyperthermia combined with intravesical chemotherapy.
Hyperthermia
The potential value of hyperthermia in treating cancer was first suggested in 1866 by a German physician after he noted disappearance of a sarcoma with a high fever due to erysipelas. The use of hyperthermia in the management of bladder cancer had not received much attention until halfway through the 20th century. During this period, hyperthermia was tested as a solitary treatment by recirculating hot fluid in the bladder. It proved to be marginally effective in reaching complete response (CR) even with temperatures of up to 80 °C [Citation5]. Therefore, combinations of hyperthermia with radio- or chemotherapy were explored.
Techniques of establishing intravesical hyperthermia have developed since. At present, four main techniques exist. All of these combine hyperthermia with intravesical chemotherapy. The first technique makes use of the before-mentioned recirculation of fluid heated outside of the body, such as the UniThermia [Citation6] or Combat BRS systems [Citation7] do. Secondly, a relatively new technique which is still in the pre-clinical phase uses magnetic fluid in combination with magnetic resonance for heating [Citation8]. The third and fourth more extensively investigated techniques make use of radiofrequency (RF)-induced hyperthermia. This can be applied from outside of the body (deep external hyperthermia, e.g. a BSD-2000 device [Citation9]) or, as is discussed in this manuscript, with an intravesical RF-emitting antenna (Synergo® [Citation10]) device.
The goal of this review is to provide an overview and reflection on the current status of RF-induced chemo-hyperthermia (CHT). Since a systematic review covering literature up to January 2011 exists [Citation11], we intend to give an update based on the newly acquired knowledge.
Evidence acquisition and synthesis
A literature search in MEDLINE, Embase and Cochrane databases was performed on 30 September 2015, resulting in papers published from 1950 to 29 September 2015. Key search terms used (as well as synonyms, abbreviations, and entry terms/MeSH terms) were ‘bladder cancer’, ‘hyperthermia’, ‘chemohyperthermia’, and ‘radiofrequency’. Only titles and abstracts were searched. Searches with these key terms were combined with the appropriate Boolean operators. Relevant conference abstracts were also searched for manually. ClinicalTrials.gov identified only one additional relevant study regarding overactive bladder symptoms after Synergo® treatment which is currently recruiting.
Although this review is not a systematic review, it is based on a very sensitive search. Titles and abstracts of the search result were subsequently scanned for relevance. Where applicable, authors and experts were asked for additional information. Manuscripts were selected for further review if an English abstract was available, if it concerned RF-induced CHT, or in some cases, if it concerned intravesical hyperthermia with a very similar rationale such as conductive heating or deep external RF-induced hyperthermia.
Clinical trials regarding intravesical RF-induced CHT were evaluated and ordered in an overview to facilitate a comprehensive comparison. This overview focuses on clinical trials published after the systematic review of Lammers et al., and thus can be seen as a sequel of the table presented in that publication [Citation11].
No meta-analysis was performed because the newly published manuscripts since January 2011 did not compare RF-induced CHT with other treatments, or were too few for pooling data [Citation11]. Since most chemo-hyperthermia treatments are performed with mitomycin C (MMC), the abbreviation CHT in this manuscript is used for the combination of hyperthermia with MMC unless otherwise stated.
Technical implementation (the Synergo® device)
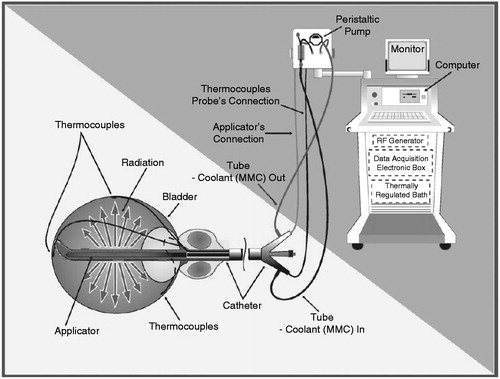
The only currently available device applying intravesical RF-induced hyperthermia is the Synergo® system, which has been described in detail previously [Citation10]. In short, local hyperthermia is accomplished by a 915-MHz intravesical microwave applicator heating the bladder wall. This applicator is located in the distal end of a three-way transurethral 20F catheter with Tiemann tip, containing a lumen for inflation of the balloon, a lumen for fluid introduction, and another lumen for outflow of fluid. Three thermocouples are tangentially distended from the catheter tip to measure temperature at the bladder neck, and the dorsal and lateral bladder walls. Two additional thermocouples are incorporated more distantly from the tip, measuring temperature in the proximal urethra ().
A bladder wall temperature between 41–44 °C is preferable [Citation12]. Temperatures and RF power are monitored and regulated on an external computerised unit to which the catheter is connected, resulting in a closed circuit. This unit also regulates the speed of the peristaltic pump and the cooling of the fluid being pumped into the bladder. The latter is done for urethral thermal protection.
Due to its favourable safety profile and the substantial experience in combination with hyperthermia, MMC is being used as the drug of first choice in chemo-hyperthermia treatment. If MMC is not an option, epirubicin has been accepted as a safe and efficient alternative. Typically, treatment is given in two sessions of 20–30 min in which the aim is to achieve a temperature above 41 °C during at least 20 min per session. Chemo-hyperthermia is most commonly applied in a prophylactic protocol of 2 × 20 mg MMC or 2 × 25 mg epirubicin, also described as an adjuvant treatment, or alternatively in an ablative protocol of 2 × 40 mg MMC or 2 × 50 mg epirubicin, also known as neo-adjuvant treatment. Dosages are dissolved in 50–60 mL of saline or distilled water. Reasons for changing the instilled fluid after 20–30 min are dilution by newly produced urine and serum exudation, disintegration of the solution, and that MMC absorption occurs mainly during the first 22 min [Citation6,Citation13]. After initial catheter insertion the bladder is emptied. For minimisation of drug dilution patients are instructed to limit fluid intake as much as possible before each treatment.
For induction 6–8 weekly instillations are administered, after which a maintenance period follows consisting of instillations every 4–6 weeks for up to a year. During the second year the interval is widened if no recurrence or progression was seen. Follow-up cystoscopies are usually performed every 3 months. Strictly taken, treatment can be stopped after a disease-free period of 2 years, although no studies have been performed to find the optimal treatment duration. Nonetheless, many patients in our centres continue treatment with longer intervals after a period of 2 years from the first instillation.
Rationale and assumed mechanism
Although some in vitro and in vivo studies have been performed to unravel the mechanism through which hyperthermia contributes to the anti-tumour effect, no uniform theory yet exists. Nonetheless, results give direction to the rationale for using hyperthermia in combination with intravesical chemotherapy.
When hyperthermia alone is applied, this results in direct damage to the DNA, RNA and proteins, and additionally inhibits their synthesis resulting in a decreased proliferation rate [Citation4,Citation7,Citation14–16]. If subsequently repair mechanisms fail due to altered intracellular metabolism, cell death and apoptosis are accelerated [Citation17]. These changes can be seen from temperatures of 40.5 °C and beyond, with magnitude inclining with temperature [Citation4,Citation18]. Due to a change in intracellular metabolism and resulting acidic and hypoxic micro-environment, a further sensitisation to both hyperthermia and cytotoxic agents occurs. Beside these direct effects, the immune system seems to be boosted [Citation4,Citation19], resulting in increased cellular immune response and autovaccination against tumour cells. Lastly, hyperthermia may increase blood flow in normal surrounding tissue due to vasodilatation, increasing MMC exposure. However, tumour vasculature is less responsive and thus less efficient in dissipating heat, resulting in a higher local temperature than in non-cancerous cells. This possibly results in a higher treatment specificity for cancerous cells. The same seems to be the case at cellular level, since normal urothelial cells demonstrate more resistance to CHT [Citation15,Citation17,Citation20,Citation21], although conflicting results are reported [Citation22].
By introducing chemotherapy to hyperthermia, this additionally damages the vital cellular molecules in the absence of a properly functioning repair mechanism, resulting in an otherwise absent clinically relevant effect [Citation16,Citation17]. Thus, hyperthermia leads to drug potentiation. This is demonstrated by several in vitro studies which show a synergistic instead of just an additive effect on cytotoxicity [Citation18,Citation23]. A possible role in this synergy exists for p53 [Citation16,Citation17] and heat-shock proteins [Citation4]. Lastly, an increased tissue and cellular membrane penetration is presumed but has not yet been confirmed.
Although an increased penetration is an intuitive hypothesis, Wallner et al. [Citation24] found that hyperthermic potentiation of MMC activity was maximal if heating was done simultaneously to exposure, or up to 3 h following MMC exposure. The fact that heating cells after MMC has been removed from the medium still potentiates MMC cytotoxicity argues against the importance of permeability or activation changes at elevated temperatures; rather, it points to the importance of alterations in damage repair processes. Moreover, RF-induced CHT does not result in toxic plasma levels which might be expected in increased tissue penetration [Citation13].
At present, reason for choosing intravesical RF-induced CHT instead of other ways of heating is the assumption that a more controlled and effective heating, including a more precise temperature measurement can be achieved, although no good comparative studies have been performed. RF-induced CHT has the advantage of being the most extensively investigated form of applying intravesical hyperthermia, and thus has a broader base of evidence. Fortunately, studies regarding other forms of chemo-hyperthermia are emerging.
Clinical indications
Currently, RF-induced CHT can be discussed in NMIBC patients at intermediate to high risk for recurrence and progression. Roughly, three main indications exist, namely 1) highly recurrent NMIBC under standard therapy, 2) therapy-resistant CIS or other NMIBC, which means the tumour is BCG-refractory, and severe co-morbidity or the explicit desire of the patient for bladder sparing treatments, and 3) contra-indications for BCG. As already mentioned in the introduction, these indications have been extended temporarily to overlap the indications for BCG (high risk disease) in light of the current BCG-shortage. Patients should not be given RF-induced CHT if bladder volume is less than 150 cm3 (severe bladder spasms and pain), if urinary bladder diverticulum with a cumulative diameter of larger than 1 cm is present (risk of perforation and MMC diffusion intraperitoneally), or if catheterisation problems are expected.
The setting of RF-induced CHT can be prevention of tumour recurrence (prophylactic, or adjuvant therapy), or eradication of bladder tumour (ablative, or neo-adjuvant therapy). Both have been used in studies, and results are promising (described below).
Efficacy
Chemo-hyperthermia alone
Only a few comparative studies of RF-induced CHT with MMC alone or with BCG exist. Most papers describe experience with CHT alone, as a single-arm study. Nonetheless, this does provide an impression of its general efficacy. Regarding recurrence, for example, rates vary from 16–89%, see also in this paper as well as from the systematic review of Lammers et al. [Citation11,Citation25–33]. Two outliers are seen in this range, both representing studies with patients not suitable and/or unwilling for cystectomy [Citation29,Citation34]. If these are excluded, a recurrence rate of 16∼50% remains (22.6∼50% if follow-up is at least 2 years). Moreover, recurrence rates in the earliest studies are different from the later studies performed, since the principal exploratory studies all made use of CR or partial response scoring with subsequent recurrence rates, regardless of dose and treatment schedule [Citation10,Citation35], whereas later studies used specific ablative or prophylactic treatment protocols.
Table 1. Overview of all clinical trials since January 2011 concerning initial response, recurrence and progression chances after RF-induced chemo-hyperthermia in non-muscle-invasive bladder cancer patients.
Progression is reported less often than recurrence, since it requires a longer follow-up of NMIBC. Nonetheless, some figures are published: overall 0–36% show progression, and when only studies with a follow-up of >2 years are considered, the same figures apply [Citation11,Citation25–33]. Most of these studies define progression as occurrence of MIBC. Remarkable is the wide spread of progression rate, mostly caused by the results of more recent publications [Citation29,Citation30,Citation32]. These papers have a definition of progression which is much broader than just the occurrence of MIBC. Sooriakumaran et al. [Citation30], for example, also include in the definition patients with initiation of further treatment (BCG, cystectomy, radiotherapy, diverticulectomy), extravesical disease, or distant metastases, reaching a progression rate of 36%. The study of Kiss et al. used a cohort of patients who were not fit for, or had refused cystectomy. Moreover, most patients did not complete the treatment protocol due to adverse events [Citation29]. If these studies are excluded, a progression rate of 0–11.9% remains [Citation11,Citation25–28,Citation33].
Most studies published allow comparison of prophylactic therapy with\ ablative therapy. CR after ablative therapy ranges from 42–85.5%, with subsequent recurrence rates of 16–30.4% (follow-up ranging from 16–38 months). Progression rate for complete responders varies from 0% to 27% [Citation11,Citation25,Citation26,Citation30–32]. See also . For prophylactic therapy, recurrence rate varies from 15.6–56% and progression rate from 0–11.9% (follow-up ranging from 10–38 months) [Citation11,Citation25–28,Citation31,Citation33].
Although results in patients that have previously failed intravesical therapy for NMIBC are contradictory, some patients do seem to have additional benefit from CHT treatment. For example, Lammers et al. [Citation11] report on a multicentre Italian project in which patients who have been treated de novo with CHT do significantly better than those who had previous intravesical instillations (2-year recurrence-free survival (RFS) of 91% and 62% respectively, p = 0.006). This finding is supported by a previous study showing a RFS of 75.4–58.8%, respectively [Citation36]. However, in a different paper no difference in response rate was seen in CIS patients failing or responding to BCG [Citation37]. Also in intermediate and high risk patients in general, studies show no effect of previous BCG failure [Citation38,Citation39]. Arends et al. describe their 10-year experience in a cohort of 160 patients in which 81% of the subjects was previously treated with BCG. Although not analysed separately, the overall 2-year RFS was 47% [Citation28]. Thus, a substantial proportion of pre-treated patients still benefits from treatment.
Bladder preservation rates have been described previously, reaching a pooled rate of 89.4% [Citation11]. In agreement with the previous literature, more recent studies vary from 71–100% in bladder preservation rate, depending on the treatment protocol used [Citation25,Citation27,Citation29–32].
Of further interest, Arends et al. evaluated the difference in efficacy between MMC and epirubicin, both in a prophylactic and an ablative protocol [Citation28]. They found a pooled 2-year RFS of 46% and 55% for MMC and epirubicin, respectively (p = 0.30). No other groups have investigated efficacy of chemotherapeutics other than MMC in CHT [Citation17].
It needs to be noted that in all the above-mentioned rates, a high level of methodological heterogeneity (mostly in patient characteristics, treatment schedule and follow-up time) exists, hampering the generalisability of these figures. Therefore, cautious interpretation is warranted.
Chemo-hyperthermia vs. MMC alone
Up to this moment, four studies have been performed comparing RF-induced CHT versus MMC alone. All four were performed by the same author (although patients did not overlap according to the author except for the long-term results paper) and have been clustered in a meta-analysis in a recent systematic review [Citation11,Citation20,Citation21,Citation40,Citation41]. However, several drawbacks are presented. One of these studies was a non-randomised study; allocation in three treatment groups (i.e. CHT, MMC alone, or EMDA) was according to patient preference [Citation40]. Moreover, different treatment duration and drug concentrations were used. Three out of four studies were performed in intermediate to high risk NMIBC patients, the fourth in single, small (<2 cm) stage Ta/T1 G1–2 patients.
In total, 26/93 patients (28%) had a recurrence in the clustered CHT group, vs. 67/99 (67.7%) in the MMC alone group. After meta-analysis this was corrected for different weighting of studies, resulting in an overall risk ratio of 0.410 (95% CI, 0.290–0.579), indicating 59% less risk of recurrence after CHT compared to MMC alone [Citation11]. Although heterogeneity in the studied populations and methodological shortcomings are present, this outcome does indicate a substantial benefit of CHT over MMC alone.
CR rates were only reported in two studies comparing CHT with MMC alone, showing 66% in both for CHT, vs. 22% (p = 0.001) and 27.7% (p-value not reported) in MMC alone [Citation20,Citation40]. Progression, bladder preservation, and overall survival were derived from two studies using the same cohort, in which the latest paper reports the long-term outcomes of the same intermediate to high risk patient cohort [Citation21,Citation41]. Over a median follow-up of 90 months (range 6–154), 2/35 patients (5.7%) treated with CHT and 3/40 (7.5%) treated with MMC alone progressed. Bladder was preserved in 86.1% vs. 78.9%, respectively (p = 0.129), and overall survival was 29/35 (82.9%) vs. 31/40 patients (77.5%), respectively (p = 0.558). No disease-specific deaths occurred.
Chemo-hyperthermia vs. BCG
Until now, no publications on the comparison of RF-induced CHT vs. BCG exist, although an abstract of Arends et al. [Citation33] was presented at the latest conference of the European Association of Urology (EAU) in 2015. In this abstract a randomised controlled clinical trial comparing 1 year of RF-induced CHT with 1 year of BCG in the adjuvant setting was discussed. The study population consisted of 190 patients with intermediate to high risk NMIBC, recruited over 11 centres between 2002 and 2012. A 24-month RFS of 81.8% in the RF-induced CHT group, compared to 64.8% in the BCG group was found on the per-protocol (PP) analysis (p = 0.0216). The intention-to-treat analysis, however, did not result in a significant difference (78.1% vs. 64.8% respectively, p = 0.0803). In a specific analysis for CIS patients only, a CR rate of 88.9% and 85.7% was found for CHT and BCG, respectively (p > 0.1). Progression was described in less than 3% in both groups (personal communications).
Another interesting yet unpublished project is the HYMN trial (ClinicalTrials.gov; NCT01094964). In this two-arm, randomised, open-label and multicentre study from the UK, RF-induced CHT is compared with BCG or standard therapy as a second-line therapy in 104 patients with recurrent NMIBC after a first-line induction or maintenance BCG treatment. Whether a patient receives BCG maintenance or standard therapy depends on the response to the first-line BCG treatment. Primary outcomes were disease-free survival time (DFS) in all patients, and CR rate at 3 months in patients with CIS. Secondary outcomes will be RFS, progression-free survival, overall survival (OS), disease-specific survival (DSS), safety and tolerability, quality of life, and cost effectiveness. Lastly, biomarkers of response to standard and investigational treatment will be assessed. An abstract was presented recently showing no difference regarding CR and overall DFS [Citation42]. The DFS in patients with papillary disease alone at randomisation (n = 33), however, had an improved DFS for RF-induced CHT compared to BCG or standard therapy (HR 0.40, 95% CI 0.16–0.98, p = 0.05), whereas with any CIS at randomisation (n = 71), DFS was worse (hazard ratio (HR) 2.17, 95% confidence interval (CI) 1.15–4.08, p = 0.02). No difference in adverse events was found.
The concept of CHT with a different technique has been compared with BCG solely by Ekin et al. [Citation43]. They compared conductive CHT (n = 40) with BCG (n = 142) in a retrospective propensity score-matched study in high risk NMIBC patients and found a 2-year RFS in conductive CHT and BCG groups of 76.2% and 93.9%, respectively (p = 0.020). Conductive CHT treatment (HR 5.42; 95% CI 1.11–26.43; p = 0.036) and high-grade tumour (HR 4.60; 95% CI 1.01–20.88; p = 0.048) were associated with an elevated odds of tumour recurrence. In multivariate Cox regression analysis, there was no significant difference between conductive CHT and BCG in the odds of recurrence (p = 0.054). There were no differences in progression.
Chemo-hyperthermia vs. radical cystectomy
Radiofrequency-induced CHT has also been compared with the other gold standard, i.e. radical cystectomy (RC), by Nair et al. [Citation44]. This prospective single-centre review matched 103 high risk NMIBC patients treated with RF-induced CHT to 51 patients undergoing a RC. Patients in the CHT group had a higher mean age and were more frequently treated with BCG before therapy. Results are only available in an abstract, showing a 5-year DSS of 85.2% and 74.6% in the CHT and RC cohorts, respectively, and an OS of 61.9% vs. 68.4%. For survival rates, a median follow-up of 40 months (4–92 months) was accomplished. More complications (Clavian-Dindo score >2) were seen after RC (21%) compared to CHT (0%), as well as more deaths (90-day mortality of 4% vs. 0%, respectively). These results seem to promote CHT over RC, although it needs to be noted that this is not a randomised controlled and blinded trial, but a matched design in which patient groups might have differed significantly. Moreover, results regarding deaths and complications are not expected to be worse in CHT with this short follow-up anyway.
Chemo-hyperthermia long-term efficacy
Just as studies comparing CHT with BCG are needed, studies regarding long-term effects of RF-induced CHT are too. A few studies have been performed with a median follow-up of at least 60 months (5 years, ) [Citation28,Citation29,Citation32,Citation41], although only two of these studies were designed to study long-term efficacy [Citation32]. Colombo et al. [Citation41] described an estimated 10-year DFS of 52.8% for RF-induced CHT compared to 14.6% for MMC alone (p < 0.0001). The estimated 5-year DFS for CHT and MMC alone was 61.7% and 21.3%, respectively. Both progression and RC rates did not differ significantly between treatment groups (CHT 2/35 and 1/35, respectively; RC 3/40 and 3/40, respectively).
Kiss et al. [Citation29] had a median follow-up of 50 months, although efficacy analysis was hampered by the high fall out rate due to severe adverse events. They reported 6/21 patients (29%) without recurrence after this follow-up period. Also 6/21 patients underwent a cystectomy. Regarding mortality, 2/21 died due to metastatic disease, and 5/21 due to other non-cancer-related causes. Progression was not specified. At the EAU conference 2015, Lüdecke et al. [Citation32] presented their data regarding long-term efficacy of ablative RF-induced CHT in 271 high risk NMIBC patients from 7 centres, with a median follow-up of only 2.2 years (28 days–12.9 years), but in some patients up to 12.9 years. CR was achieved in 76.1%, and 76.8% remained tumour free for a mean of 28 months (2.4 months–10.8 years). Halachmi et al. [Citation38], with a retrospective study in 56 T1G3 NMIBC patients, found a 4-year recurrence chance of 51%.
Last but not least, an as yet unpublished Italian multicentre, non-randomised, patient-for-patient matched study was promoted and sponsored by the health provider (Lombardy Region Health Care System) in order to compare the outcomes of patients receiving adjuvant RF-induced CHT or the current treatment according to the EAU guidelines. Between January 2005 and July 2011 consecutive patients were recruited into the treatment arm at 6 centres while for the control arm the archived records of patients treated at a single centre between January 2005 and December 2008 were used. Only intermediate or high risk patients according to the EORTC tables were accepted for comparative analysis. In all, 189 RF-induced CHT and 180 control patients were available for the analysis of outcomes. At a minimum of 24 months of follow-up, 24.9% and 38.3% of patients experienced a recurrence in the RF-induced CHT and in the control arm, respectively (p < 0.006). During the whole follow-up period (median of 55.3 months), a sixfold rate of radical cystectomies was registered in the control group compared to the RF-induced CHT arm (20 and 3 cystectomies, respectively; p = 0.0015) (Lombardia project, unpublished data, R. Colombo, Milan, Italy).
Adverse events
An important factor in determining the value of RF-induced CHT is the type and quantity of adverse events (AEs). For RF-induced CHT, these can be divided into AEs seen during treatment and AEs which prolong after treatment. Most frequently seen AEs during treatment consist of bladder spasms and pain (21.6% and 17.5%, respectively) [Citation11]. Lower urinary tract symptoms (LUTS) of storage (frequency, dysuria, urgency, nocturia) (25.6%) and haematuria (6.0%) are the AEs most commonly seen after treatment. Other commonly observed AEs include cystitis-like symptoms (definitions vary greatly, and overlap with LUTS) and allergic reactions/rash (0–24%, with rashes included in the higher percentage). Urethral strictures occur in only 0–10% of cases, with 3.5% being mentioned in the recent systematic review [Citation11,Citation25–33]. Most adverse events are reported to be self-limiting and reversible. Adverse event related drop-out rate is about 3.8% [Citation11], although one study reported a substantially higher drop-out rate of 38%. In this study not all patients were fit for or had refused cystectomy, implying that this was a highly selected cohort with possibly a worse starting point [Citation29].
During follow-up cystoscopy, an asymptomatic posterior wall thermal reaction (PWTR) can be seen in almost all patients [Citation11,Citation28]. Histological damage mainly occurs in the tumour tissue, though leaving normal epithelium relatively unharmed [Citation35]. In the study by Rath-Wolfson et al. [Citation45] using anaesthetised sheep, histological changes in both RF-induced CHT and control groups showed foci of oedema and haemorrhage with inflammation in the lamina propria and serosa. Foci of desquamation of the epithelium were noticed in the treated sheep. Histological analysis of the treated group showed no significant differences compared to the control group.
Influence on endocrine homeostasis has also been tested, in 11 patients receiving external RF-induced CHT, showing none of the tested hormones (i.e. the thyroid, cortisol and sex hormones) to be altered significantly [Citation46]. Moreover, Paroni et al. [Citation13] investigated the plasma MMC concentrations after 20 mg and 40 mg of MMC with CHT, vs. both concentrations without hyperthermia. They found a maximum plasma concentration of 67.9 ng/mL after 45–60 min of treatment, which was seen in the 40 mg MMC with CHT group. Median value of systemic absorption for this group was 19.4 ng/mL, compared to 5.56 ng/mL in the 20 mg MMC with CHT-group. These values were significantly higher than those after MMC alone with the same dose. All measured plasma concentrations were well below the threshold for myelosuppression (i.e. 400 ng/mL).
In comparison with MMC instillations, and based on the four previously mentioned papers comparing MMC alone to RF-induced CHT, local toxicity was slightly higher in CHT, although the difference was not significant [Citation20,Citation21,Citation40,Citation41]. Only one of these four studies found significantly more pelvic pain and PWTR in the CHT group [Citation21].
When comparing RF-induced CHT with BCG instillation, more allergic reactions, pain, bladder spasm, strictures, catheter issues and PWTR are found for CHT, compared to more fever, fatigue, arthralgia, haematuria, incontinence and frequency in BCG treatment [Citation33].
Altogether, it seems that toxicity after CHT is higher than after MMC alone, whereas inadequate reporting hampers comparison with other treatments. Based on just one conference abstract, toxicity in CHT compared to BCG is not necessarily higher, but is mainly characterised by different, instead of more frequent, AEs.
Critical appraisal and future perspectives
With 15 years of experience, RF-induced CHT has become a commonly applied treatment modality in our centres. Nonetheless, few properly designed randomised, controlled clinical trials have been performed to date. As a consequence, high level evidence for efficacy and adverse events remains limitedly available and RF-induced CHT is still considered an experimental form of therapy. This is one of the reasons why centres hesitate to use this modality. Randomised controlled clinical trials are being executed though, with some being submitted and others being presented at conferences (which are described earlier in this review).
Another conceivable reason for reservations regarding intravesical RF-induced CHT could be the amount of adverse events seen during and after treatment. However, within the current indications, patients often have BCG or radical cystectomy as the only alternatives. Logically, adverse events and complications due to radical cystectomy are more severe than in the case of RF-induced CHT [Citation44]. Regarding BCG instillations, patients suitable for RF-induced CHT often failed BCG or were BCG-intolerant. In deciding which treatment modality should be used, one must be conscious of the, albeit different, potentially serious or frequent adverse events of BCG.
Costs of intravesical RF-induced CHT are regarded as being substantial, although previously mentioned costs in other papers are outdated [Citation47]. One study on conductive heating with the UniThermia device mentions device costs of about €42 000 compared to €90 000 for the Synergo® console. Disposable UniThermia catheters cost about €200 each, compared to €600 for the three-way catheters used in the treatment with Synergo® [Citation6]. However, this estimation for Synergo® costs is too high. Total costs per treatment vary from about €650 to €850 in our centres, depending on the drug and dose used (MMC or epirubicin), and include dispersed acquisition costs.
No studies on cost-effectiveness in RF-induced CHT have yet been published. However, the Lombardia project mentioned above attempted for the first time to assess the cost–benefit ratio of RF-induced CHT. The cost-related model was designed in order to simulate treatment outcome and cost in 100 patient pairs over a period of 10 years. One of the patients in each pair was treated by RF-induced CHT and the second according to current EAU guideline recommendations. Based on this model, RF-induced CHT is expected to reduce the overall cost for treatment of intermediate and high risk NMIBC at long-term (5-10 years) follow-up compared to current treatments. The cost/effectiveness of RF-induced CHT treatment is currently under evaluation from the health provider in view of its possible total reimbursement in the routine clinical practice (Lombardia project, unpublished data, R. Colombo, Milan, Italy).
Besides costs, some important obscurities in current knowledge remain. For starters, it would be informative to have data on the effect on quality of life in CHT-treated patients compared to BCG or MMC treated, or even radical cystectomised, patients. As written in the Evidence acquisition and synthesis section above, most of the research has been performed with MMC. Epirubicin has been described in only a few studies, and no good comparison with MMC was possible [Citation28]. Moreover, no evidence yet exists regarding treatment duration. Although consensus exists for starting treatment with an induction course and subsequently giving a maintenance scheme of a combined total of 2 years, many patients are treated for longer periods [Citation11]. Studies addressing all these issues would be most valuable.
Studies on different forms of achieving hyperthermia are being performed, and authors have been increasingly publishing their results in the past 2 years. Almost all make use of the conductive heating technique, reaching an efficacy of 18–41% for recurrence rates, 10.3–18% for progression rates, and have about 50% of grade 2 adverse events, with 9.2–14.3% showing grade 3 AEs (Common Terminology Criteria for Adverse Events (CTCAE) version 6) [Citation6,Citation7,Citation43,Citation48]. Nonetheless, comparative studies of RF-induced CHT with these new forms of (conductive) heating are important for determination of the superior hyperthermic treatment in the future.
The place of RF-induced CHT in future NMIBC management will partly be shaped by conclusions on the above-mentioned issues. However, more ways of improving pharmacokinetics, drug exposure and pharmacodynamics will likely have an important share as well.
Conclusion
Radiofrequency-induced chemo-hyperthermia remains a promising treatment modality which appears to be a potential alternative to BCG immunotherapy. It can also be used in patients unfit to undergo radical cystectomy, or to prolong bladder preservation time. Adverse events and safety profile seem to be acceptable in the context of the indication for RF-induced CHT. Nonetheless, a more solid scientific basis is needed to define the position of RF-induced CHT in relation to other hyperthermic treatment modalities and in relation to the newly emerged non-hyperthermia treatments.
Declaration of interest
J.A.W. is an investigator for Medical Enterprise Ltd (MEL), Amsterdam, without financial compensation and no conflict of interest, and was an adviser for MEL Amsterdam in 2014, with financial compensation and no conflict of interest. The other authors have no other interests to declare. The authors alone are responsible for the content and writing of the paper.
References
- Babjuk M, Böhle A, Burger M, Compérat E, Kaasinen E, Palou J, et al. Guidelines on non-muscle-invasive bladder cancer (Ta, T1 and CIS). Eur Urol 2015. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Non-muscle-invasive-Bladder-Cancer-2015-v1.pdf
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol 2006;49:466–75; discussion 475–7
- Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Pineiro L, Ojea A, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette–Guerin: External validation of the EORTC risk tables. Eur Urol 2011;60:423–30
- Slater SE, Patel P, Viney R, Foster M, Porfiri E, James ND, et al. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non-muscle invasive bladder cancer. Ann R Coll Surg Engl 2014;96:415–19
- England HR, Anderson JD, Minasian H, Marshall VR, Molland EA, Blandy JP. The therapeutic application of hyperthermia in the bladder. Br J Urol 1975;47:849–52
- Soria F, Milla P, Fiorito C, Pisano F, Sogni F, Di Marco M, et al. Efficacy and safety of a new device for intravesical thermochemotherapy in non-grade 3 BCG recurrent NMIBC: A phase I–II study. World J Urol 2015. doi: 10.1007/s00345-015-1595-3. Epub 2015 May 31
- Sousa A, Inman BA, Pineiro I, Monserrat V, Perez A, Aparici V, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia 2014;30:166–70
- Oliveira TR, Stauffer PR, Lee CT, Landon CD, Etienne W, Ashcraft KA, et al. Magnetic fluid hyperthermia for bladder cancer: A preclinical dosimetry study. Int J Hyperthermia 2013;29:835–44
- Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171–5
- Colombo R, Lev A, Da Pozzo LF, Freschi M, Gallus G, Rigatti P. A new approach using local combined microwave hyperthermia and chemotherapy in superficial transitional bladder carcinoma treatment. J Urol 1995;153:959–63
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: A systematic review. Eur Urol 2011;60:81–93
- Mazzoleni F, Albo G, Verweij F, Botteri E, Detti S, Colombo R, et al. Thermo-chemotherapy for superficial transitional cell carcinoma of the bladder. Results of a multicentric clinical study: ‘Synergo lombardia’. Anticancer Res 2010;30:1538–9
- Paroni R, Salonia A, Lev A, Da Pozzo LF, Cighetti G, Montorsi F, et al. Effect of local hyperthermia of the bladder on mitomycin C pharmacokinetics during intravesical chemotherapy for the treatment of superficial transitional cell carcinoma. Br J Clin Pharmacol 2001;52:273–8
- Haveman J, Smals OA, Rodermond HM. Effects of hyperthermia on the rat bladder: A pre-clinical study on thermometry and functional damage after treatment. Int J Hyperthermia 2003;19:45–57
- Matzkin H, Rangel MC, Soloway MS. In vitro study of the effect of hyperthermia on normal bladder cell line and on five different transitional cell carcinoma cell lines. J Urol 1992;147:1671–4
- van der Heijden AG, Hulsbergen-Van de Kaa CA, Witjes JA. The influence of thermo-chemotherapy on bladder tumours: An immunohistochemical analysis. World J Urol 2007;25:303–8
- van der Heijden AG, Verhaegh G, Jansen CF, Schalken JA, Witjes JA. Effect of hyperthermia on the cytotoxicity of 4 chemotherapeutic agents currently used for the treatment of transitional cell carcinoma of the bladder: An in vitro study. J Urol 2005;173:1375–80
- Uchibayashi T, Lee SW, Kunimi K, Ohkawa M, Endo Y, Noguchi M, et al. Studies of effects of anticancer agents in combination with/without hyperthermia on metastasized human bladder cancer cells in chick embryos using the polymerase chain reaction technique. Cancer Chemother Pharmacol 1994;35:S84–7
- Arends TJ, Falke J, Lammers RJ, Somford DM, Hendriks JC, de Weijert MC, et al. Urinary cytokines in patients treated with intravesical mitomycin-C with and without hyperthermia. World J Urol 2015;33:1411–17
- Colombo R, Da Pozzo LF, Lev A, Freschi M, Gallus G, Rigatti P. Neoadjuvant combined microwave induced local hyperthermia and topical chemotherapy vs. chemotherapy alone for superficial bladder cancer. J Urol 1996;155:1227–32
- Colombo R, Salonia A, Da Pozzo LF, Naspro R, Freschi M, Paroni R, et al. Combination of intravesical chemotherapy and hyperthermia for the treatment of superficial bladder cancer: Preliminary clinical experience. Crit Rev Oncol Hematol 2003;47:127–39
- Farr S, Chess-Williams R, McDermott C. Selective cytotoxicity of gemcitabine on superficial malignant vs normal human urothelial cells and the effects of hyperthermia. Asia Pac J Clin Oncol 2014;10:131
- Mauroy B, Bonnal JL, Prevost B, Chive M, Lhotellier V, Sozanski JP, et al. Etude de la synergie hyperthermie micro-onde/chimiotherapie intravesicale dans la prevention des recidives des tumeurs superficielles de vessie. [Study of the synergy of microwave hyperthermia/intravesical chemotherapy in the prevention of recurrences of superficial tumours of the bladder]. Prog Urol 1999;9:69–80
- Wallner KE, Banda M, Li GC. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer Res 1987;47:1308–12
- Moskovitz B, Halachmi S, Moskovitz M, Nativ O, Nativ O. 10-year single-center experience of combined intravesical chemohyperthermia for nonmuscle invasive bladder cancer. Future Oncol 2012;8:1041–9
- Volpe A, Racioppi M, Bongiovanni L, D’Agostino D, Totaro A, D’Addessi A, et al. Thermochemotherapy for non-muscle-invasive bladder cancer: Is there a chance to avoid early cystectomy? Urol Int 2012;89:311–18
- Maffezzini M, Campodonico F, Canepa G, Manuputty EE, Tamagno S, Puntoni M. Intravesical mitomycin C combined with local microwave hyperthermia in non-muscle-invasive bladder cancer with increased European Organization for Research and Treatment of Cancer (EORTC) score risk of recurrence and progression. Cancer Chemother Pharmacol 2014;73:925–30
- Arends TJ, van der Heijden AG, Witjes JA. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J Urol 2014;192:708–13
- Kiss B, Schneider S, Thalmann GN, Roth B. Is thermochemotherapy with the Synergo system a viable treatment option in patients with recurrent non-muscle-invasive bladder cancer? Int J Urol 2015;22:158–62
- Sooriakumaran P, Chiocchia V, Dutton S, Pai A, Ayres BE, Le Roux P, et al. Predictive factors for time to progression after hyperthermic mitomycin C treatment for high-risk non-muscle invasive urothelial carcinoma of the bladder: An observational cohort study of 97 patients. Urol Int 2015. doi: 10.1159/000435788. Epub 2015 Aug 6
- Lüdecke G, Hasner F, Hanitzsch H, Schmidt M. The German study group of intravesical hyperthermia-chemotherapy in non-muscle-invasive bladder cancer presents their long-term results in efficacy and tolerability for optimized adjuvant therapy and bladder preservation. J Clin Oncol 2013;31:S
- Lüdecke G, Shchafer L, Nativ O, Witzsch U, Hanitzsch H, Hasner F, et al. Radiofrequence induced hyperthermia chemotherapy (RIHTC) in high-risk non-muscle invasive bladder cancer (NMIBC): Multiinstitutional, international outcome analysis of 271 treated patients with a follow-up time of more than 2 years. Eur Urol 2015;14:e949
- Arends TJH, Nativ O, Maffezzini M, De Cobelli O, Van Der Heijden AG, Witjes JA. Results of the first randomized controlled trial comparing intravesical radiofrequency induced chemohyperthermia with mitomycin-C vs. BCG for adjuvant treatment of patients with intermediate-and high-risk non-muscle invasive bladder cancer. Eur Urol 2015;14:e944
- Colombo R, Da Pozzo LF, Lev A, Salonia A, Rigatti P, Leib Z, et al. Local microwave hyperthermia and intravesical chemotherapy as bladder sparing treatment for select multifocal and unresectable superficial bladder tumors. J Urol 1998;159:783–7
- Rigatti P, Lev A, Colombo R. Combined intravesical chemotherapy with mitomycin C and local bladder microwave-induced hyperthermia as a preoperative therapy for superficial bladder tumors. A preliminary clinical study. Eur Urol 1991;20:204–10
- van der Heijden AG, Kiemeney LA, Gofrit ON, Nativ O, Sidi A, Leib Z, et al. Preliminary European results of local microwave hyperthermia and chemotherapy treatment in intermediate or high risk superficial transitional cell carcinoma of the bladder. Eur Urol 2004;46:65–71; discussion: 72
- Witjes AJ, Hendricksen K, Gofrit O, Risi O, Nativ O. Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: Experience of the European Synergo working party. World J Urol 2009;27:319–24
- Halachmi S, Moskovitz B, Maffezzini M, Conti G, Verweij F, Kedar D, et al. Intravesical mitomycin C combined with hyperthermia for patients with T1G3 transitional cell carcinoma of the bladder. Urol Oncol 2011;29:259–64
- Ayres BE, Connor A, Corbishley C, Bailey MJ. Radiofrequency hyperthermia and mitomcin C for the management of frail patients with high-risk non-muscle invasive bladder cancer who fail intravesical BCG treatment. BJU Int 2010;106:8
- Colombo R, Brausi M, Da Pozzo L, Salonia A, Montorsi F, Scattoni V, et al. Thermo-chemotherapy and electromotive drug administration of mitomycin C in superficial bladder cancer eradication. a pilot study on marker lesion. Eur Urol 2001;39:95–100
- Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int 2011;107:912–18
- Kelly JD, Buckley L, Devall AJ, Loubière LS, Barnwell JM, Feneley MR, et al. HYMN: a randomised controlled phase III trial comparing hyperthermia plus mitomycin to a second course of bacillus Calmette–Guerin (BCG) or institutional standard in patients with recurrence of non-muscle invasive bladder cancer (NMIBC) following induction or maintenance therapy. Manchester: BAUS meeting, 2015
- Ekin RG, Akarken I, Zorlu F, Tarhan H, Kucuk U, Yildirim Z, et al. Intravesical bacillus Calmette–Guerin vs. chemohyperthermia for high-risk non-muscle-invasive bladder cancer. Can Urol Assoc J 2015;9:E278–83
- Nair R, Pai A, Kaul A, Ayres B, Bailey M, Perry M, et al. Challenging the gold standard: A comparison of long-term disease specific outcomes for high-risk non-muscle invasive bladder cancer treated with mitomycin hyperthermia and radical cystectomy. Eur Urol 2014;13:e1109
- Rath-Wolfson L, Moskovitz B, Dekel Y, Kugel V, Koren R. Combined intravesical hyperthermia and mitomycin chemotherapy: A preliminary in vivo study. Int J Exp Pathol 2003;84:145–52
- Hashimoto T, Hisazumi H, Nakajima K, Matsubara F. Studies on endocrine changes induced by 8 MHz local radiofrequency hyperthermia in patients with bladder cancer. Int J Hyperthermia 1991;7:551–7
- Hasner F, Thueroff S, Chaussy C. Combined thermochemotherapy (Synergo) in non muscle invasive bladder cancer (NMIBC): 8 year follow up of a prospective monocentric cohort study. Urology 2009;74:S145
- Ekin RG, Akarken I, Cakmak O, Tarhan H, Celik O, Ilbey YO, et al. Results of intravesical chemo-hyperthermia in high-risk non-muscle invasive bladder cancer. Asian Pac J Cancer Prev 2015;16:3241–5
- Lüdecke GC, Schafer L, Weidner W, Hasner F, Hanitzsch H, Schmidt M. Organ preservation in high- and extreme high risk non-muscle-invasive bladder cancer (NMIBC): Outcome analysis of an interventional cohort study of the German Hyperthermia Chemotherapy Group in efficacy and side effects. Eur Urol 2013;12:e707–8