Abstract
Objectives The aim of this study was to investigate factors affecting ablative efficiency of high intensity focused ultrasound (HIFU) for adenomyosis. Materials and methods In all, 245 patients with adenomyosis who underwent ultrasound guided HIFU (USgHIFU) were retrospectively reviewed. All patients underwent dynamic contrast-enhanced magnetic resonance imaging (MRI) before and after HIFU treatment. The non-perfused volume (NPV) ratio, energy efficiency factor (EEF) and greyscale change were set as dependent variables, while the factors possibly affecting ablation efficiency were set as independent variables. These variables were used to build multiple regression models. Results A total of 245 patients with adenomyosis successfully completed HIFU treatment. Enhancement type on T1 weighted image (WI), abdominal wall thickness, volume of adenomyotic lesion, the number of hyperintense points, location of the uterus, and location of adenomyosis all had a linear relationship with the NPV ratio. Distance from skin to the adenomyotic lesion’s ventral side, enhancement type on T1WI, volume of adenomyotic lesion, abdominal wall thickness, and signal intensity on T2WI all had a linear relationship with EEF. Location of the uterus and abdominal wall thickness also both had a linear relationship with greyscale change. Conclusion The enhancement type on T1WI, signal intensity on T2WI, volume of adenomyosis, location of the uterus and adenomyosis, number of hyperintense points, abdominal wall thickness, and distance from the skin to the adenomyotic lesion’s ventral side can all be used as predictors of HIFU for adenomyosis.
Introduction
Adenomyosis is a common gynaecological disease in women of reproductive age [Citation1]. The reported prevalence is about 8.8–31%, and nearly two thirds of the patients are symptomatic with menorrhagia and dysmenorrhoea [Citation2,Citation3]. Treatment of adenomyosis is a clinical challenge in gynaecological practice. Traditionally, the treatment for symptomatic adenomyosis is hysterectomy, which is still the only definitive treatment for these patients [Citation4]. However, this surgical operation is unsuitable for patients who wish to keep their uterus. Conservatively, the levonorgestrel-releasing intrauterine device (IUD), gonadotropin-releasing hormone analogues (GnRHa), non-steroidal anti-inflammatory drugs (NSAIDs), and oral contraceptives are available for symptom relief. However, effects of these treatments are limited due to adverse effects, resulting in recurrence of symptoms after therapy cessation [Citation5].
Recently, ultrasound-guided high intensity focused ultrasound (USgHIFU) has been widely used for the treatment of adenomyosis [Citation6–8]. As a non-invasive technique, HIFU can selectively induce typical coagulation necrosis in adenomyotic lesions, so that the lesions lose the function of growth or bleeding and thus relieve adenomyosis-related symptoms. Many studies have found that the non-perfused volume (NPV) ratio is related to symptom relief. The greater the NPV ratio achieved, the better long-term symptom relief is sustained [Citation9,Citation10]. However, in clinical practice we found that a high NPV ratio is easy to achieve in some lesions but difficult to achieve in other lesions. To the best of our knowledge, no study has investigated the related factors that affect NPV ratios. Therefore, it is necessary to explore the factors influencing the ablative efficiency of HIFU treatment for adenomyosis.
Materials and methods
Patients
In total 245 patients (from January 2011 to July 2014) with symptomatic adenomyosis who were treated with USgHIFU in Chongqing Haifu Hospital were retrospectively reviewed. The diagnosis of adenomyosis was suspected by clinical evaluation and confirmed with ultrasound and magnetic resonance imaging (MRI). The diagnostic criteria used in this study included focal or diffuse thickening of the uterine junctional zone or the presence of a low signal intensity myometrial mass with ill-defined borders [Citation11,Citation12].
Inclusion criteria: 1) all patients had menorrhagia and/or dysmenorrhoea, 2) patients could communicate clearly with a nurse or physician during HIFU treatment, 3) no serious systemic diseases were present, 4) patients agreed to have MRI examinations 1–3 days before and 1 day after HIFU treatment.
Exclusion criteria: 1) adenomyotic lesion could not be visualised or the focus was unable to reach the adenomyotic lesion, 2) patients during pregnancy, lactation or menstruation, 3) women with suspected or confirmed uterine malignancy, 4) patients with contraindications to MRI.
Magnetic resonance imaging evaluation
Each patient underwent an MRI before and after HIFU treatment. All MRI examinations were performed with a 1.5 T Magnetom Symphony MR system (Siemens Healthcare, Erlangen, Germany). Standardised parameters performed for T1 weighted image(WI) were as follows: TR 500 ms/TE 13 ms, voxel size 1.7 × 1.3 × 5.0 mm, slice thickness 4 mm. Standardised parameters used for T2WI were as follows: TR 2300 ms/TE 100 ms, voxel size 1.0 × 1.0 × 4.0 mm, slice thickness 4 mm. Standardised parameters for contrast enhanced MRI were as follows: TR 6.23 ms/TE 2.91 ms, voxel size 1.7 × 1.2 × 4.0 mm, slice thickness 4 mm.
MR images were used to measure the thickness of the abdominal wall, the distance from the anterior surface of the lesion to the skin, the distance from the posterior surface of lesion to the sacrococcygeal surface, the volume of the adenomyotic lesion, and the maximum diameter. In addition, the location of the adenomyosis, the location of the uterus, the number of hyperintense points on T2WI (the number of hyperintense points was classified as > 5 or < 5 on the maximum level) ( and ), the signal intensity on the T2WI, and the enhancement type on the T1WI (blood supply) after intravenous injection of gadolinium were also determined through images. T2 weighted signal intensity of the lesions was compared to the myometrial signal intensity, lesions were labelled as hypointense when the signal intensity was lower than the myometrium, isointense when the signal intensity was approaching normal myometrium, and hyperintense when higher than the myometrium. Lesion enhancement was classified as mild when the enhancement was less than the myometrium, moderate when similar to the myometrium, and significant when greater than the normal myometrium ().
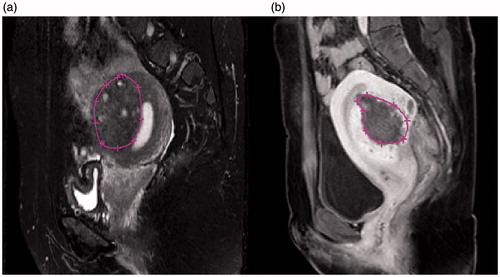
Figure 1. MRI images with and without hyperintensive points on T2WI. (a) No hyperintensive points (white dots) are present in this slice. (b) At least five hyperintensive points are shown on this slice.
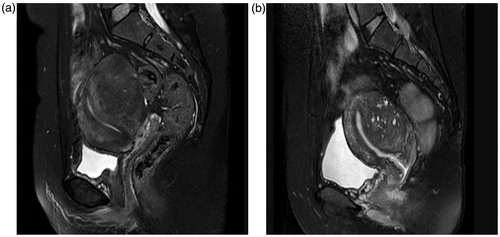
Figure 2. The measurement of the distance from the adenomyotic lesion’s ventral side to the skin, the distance of the adenomyotic lesion’s dorsal side to the sacrum and the abdominal wall thickness. (a) Abdominal wall thickness. (b) Distance from the anterior surface of adenomyotic lesion to the skin. (c) Distance from the posterior surface of adenomyotic lesion to the sacrococcygeal symphysis.
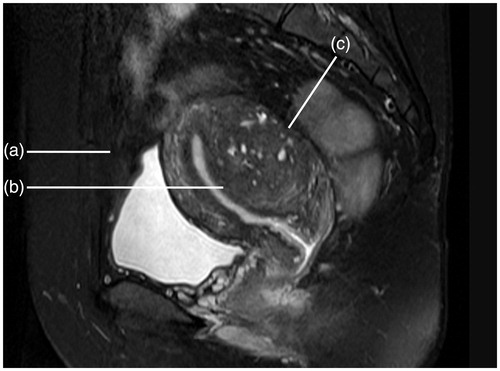
Figure 3. Contrast-enhanced types on T1WI. (a) Mild enhancement: the degree of enhancement was lower than the myometrium. (b) Moderate enhancement: the degree of enhancement was similar to the myometrium. (c) Significant enhancement: the degree of enhancement was higher than the myometrium.
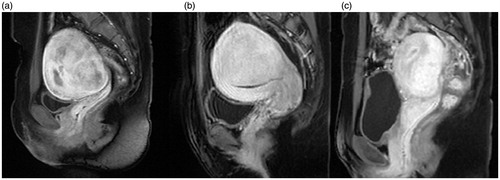
The volume of the adenomyotic lesion and the NPV were measured on each slice of the contrast-enhanced T1WI using software programmed by Chongqing Haifu Medical Technology (). The NPV ratio (defined as the non-perfused volume divided by the target adenomyotic lesion volume), the energy efficiency factor (EEF, defined as the ultrasound energy delivered for ablating 1 mm3 of the adenomyotic lesion tissue), as well as greyscale change during treatment were considered to be the key factors to evaluate ablation effect because the NPV ratio demonstrates the ablative results of HIFU treatment, the EEF shows how much energy is required for ablating 1 unit of the adenomyotic lesion in different patients, and the greyscale change during HIFU treatment indicates a quick response to HIFU. These three factors reflect the ablative efficiency of HIFU treatment.
High intensity focused ultrasound treatment
Previous studies have described the protocol of HIFU treatment for adenomyosis [Citation6,Citation7,Citation13]. All patients were required to undergo specific bowel preparation 3 days before HIFU treatment, which included ingesting liquid food for 3 days, fasting for 12 h pre-treatment and a mandatory enema in the morning of treatment day. The hair on the abdominal wall from the umbilicus to the upper margin of the pubic symphysis was shaved, and the area was then cleaned with 70% ethanol and degassed water to remove oils from the skin before treatment. A urinary catheter was inserted into the bladder to control the bladder volume and optimise the therapeutic acoustic pathway. To prevent intestinal injury, a degassed water balloon was used to compress and push away bowels from the acoustic pathway.
The procedure of USgHIFU was performed under conscious sedation using a JC or JC 200 tumour therapeutic system (Chongqing Haifu Medical Technology). An ultrasound device (MyLab 70, Esaote, Genova, Italy) was used to provide real-time imaging for monitoring and targeting the adenomyotic lesions. JC was designed for treating different types of solid tumours, including liver tumours, pancreatic cancer, bone tumours, uterine fibroids and adenomyosis, while JC 200 was mainly used to treat uterine fibroids and adenomyosis. The main difference between these two systems is that the transducer of JC has a larger movement range than that of JC 200. The patients were asked to report any discomfort and vital signs were monitored.
Every patient was carefully positioned on the HIFU treatment bed with the anterior abdominal wall in contact with degassed water. The power for point sonication was 350-400 W, and the treatment was terminated when the hyperechoic area covered the entire treated lesion or colour Doppler ultrasound imaging showed no blood supply.
Statistical methods
Normally distributed data were expressed as mean ± standard deviation. Skewed distribution of the data was indicated using the median and interquartile range. A regression analysis model was established by analysing the characteristics of the data. Linear regression analysis was used to analyse the NPV ratio and EEF, while logistic regression analysis was used for analysing the greyscale change. Statistical analysis was completed with SPSS 17.0 (IBM, Armonk, NY), and p < 0.05 was defined as statistically significant.
Results
Patients and Lesions
The average age of the 245 patients was 37.6 ± 5.1 years with a median body mass index (BMI) of 22.2 kg/m2 (interquartile range 15.0–35.0 kg/m2). The average largest diameter and the median volume of the adenomyotic lesions were 66.4 ± 17.2 mm and 89.3 mm3 (interquartile range 13.2–544.6 mm3), respectively. The mean abdominal wall thickness was 25.4 ± 8.2 mm; the mean distances from lesion ventral side to skin and dorsal side to sacrum were 53.5 ± 26.1 mm and 18.1 ± 14.4 mm, respectively ().
Table 1. Baseline characteristics of patients with adenomyosis.
HIFU ablation results
The non-perfused area was observed after HIFU treatment on contrast-enhanced MRI for every case. The median treatment time was 110 min (interquartile range 13–284 min), and the median sonication time was 18.8 min (interquartile range 1.5–64.4 min). The median sonication time per hour was 10.4 mon/h (interquartile range 3.3–26.3 min/h), the average NPV ratio was 59.7 ± 20.8% (range 5–97.7%), and the median EEF was 6.0 J/mm3 (interquartile range 0.5–78.9 J/mm3) among the 245 patients. During the treatment, significant greyscale change was observed in 175 patients (71.4%) out of 245 ().
Table 2. Treatment results of patients with adenomyosis treated by USgHIFU.
Analysis of factors influencing NPV ratio
Data were suitable for multiple linear regression analysis. The NPV ratio was used as the dependent variable. Age, BMI, position of the uterus, adenomyotic lesion, thickness of abdominal wall, volume of lesion, abdominal scar, the number of hyperintense points, signal intensity on T2WI, and enhancement type of lesion on T1WI were used as independent variables. The correlation between each of the 10 predictors and the NPV ratio was measured using a multiple linear regression analysis model one by one. Multiple linear regression analysis was conducted with a stepwise regression model. There were seven statistically significant predictors: enhancement type on T1WI, abdominal wall thickness, volume of adenomyosis, the number of hyperintense points, abdominal scar, location of adenomyosis, and the location of the uterus. The seven predictors were included one by one into the linear regression model when the p value was less than or equal to 0.05, and were eliminated when the p value was greater than or equal to 0.1. The results in show that model 7 was significantly better than the other models in the view of R2. The analysis results further show that the NPV ratio was negatively correlated with enhancement type on T1WI, abdominal wall thickness, and the number of hyperintense points, respectively. On the other hand, a positive relationship between volume of adenomyosis and NPV ratio was observed. We compared the NPV ratio of the lesions in different locations and found that the NPV ratio in the lesions at the anterior wall of the uterus was larger than the NPV ratio in the lesions at the posterior wall of the uterus, the NPV ratio in the lesions at the posterior wall of the uterus was larger than the NPV ratio in the lesions at the fundus, and the smallest NPV ratio was seen in the diffuse adenomyosis (both anterior wall and posterior wall of the uterus). The NPV ratio is also related to the location of the uterus. A higher NPV ratio is easier to achieve in adenomyotic patients with anteverted uteri, compared to retroverted uteri. The other independent variables have no significant relation with the NPV ratio ().
Table 3. The multivariable regression model* of NPV ratio. Multiple linear regression analysis was conducted with a stepwise regression model. Stepwise regression includes regression models in which the choice of predictive variables is carried out by an automatic procedure.
Table 4. Coefficient of multivariable regression modela of non-perfused volume ratio.
Relationship between the influencing factors and EEF
We considered EEF as a dependent variable, and the factors included age, BMI, abdominal wall thickness, abdominal wall scar, volume of adenomyotic lesion, the distance from the ventral side of adenomyotic lesion to skin, the distance from the dorsal side of the adenomyotic lesion to the sacrum, locations of adenomyotic lesions and location of the uterus, signal intensity on T2WI, enhancement type on T1WI set as independent variables to build a multiple regression model. The analysis results are presented in , and we found that model 7 showed the best correlation between the predictors and EEF, making it the best-fit model. Distance from the ventral side of adenomyotic lesion to the skin, enhancement type on the T1WI, volume of adenomyotic lesion, abdominal wall thickness, signal intensity on T2WI, location of adenomyotic lesions, and location of the uterus were turned into linear regression models. The results showed a positive linear relationship between the EEF and the distance from the ventral side of the adenomyotic lesion to the skin, enhancement type on T1WI, abdominal wall thickness, and signal intensity on T2WI ( and ).
Table 5. The multivariable regression model* of energy efficiency factor (EEF).
Table 6. Coefficient of multivariable regression modela of EEF.
Relationship between the influencing factors and greyscale changes
Data were divided into two sets, with greyscale change or without greyscale change. Multiple linear regression analysis was conducted with a stepwise regression model. The greyscale was regarded as a dependent variable; age, BMI, position of the uterus and adenomyotic lesion, the number of hyperintense points, thickness of abdominal wall, abdominal scar, volume of adenomyosis, signal intensity on T2WI, contrast enhancement type of lesion on T1WI were classified as independent variables. The χ2 test or Fisher’s exact analysis were used to analyse the data, which found that volume of adenomyosis, abdominal wall thickness, and the locations of adenomyotic lesions and uterus had statistically significant differences in the two sets, while others had no significant relationship with greyscale change (p > 0.05) ().
Table 7. Univariate analysis of greyscale change.
Logistic regression analysis further showed that location of the uterus and abdominal wall thickness both correlated well with greyscale change (p < 0.05). Greyscale change was more often seen in adenomyotic lesions of anteverted uterus than in a retroverted uterus. The thinner the thickness of the abdominal wall, the more easily the greyscale change was to observe. The other independent variables have no significant association with greyscale change in this multivariate analysis model ().
Table 8. Multivariate analysis of greyscale change.
Discussion
In clinical practice we found that many factors may affect the efficacy of HIFU ablation. Among these, some originated from the therapeutic device, and others were related to the characteristics of the patients. Fry et al. have demonstrated that the effect of HIFU ablation depends on the acoustic pathway and the focal field of energy deposition [Citation14]. Studies have shown that every interface in the acoustic pathway can cause absorption, reflection, and scattering of ultrasound beams. The deeper the ultrasound goes, the more acoustic energy is lost [Citation15]. This phenomenon has been confirmed by other studies. In comparison to the lesions located at the superficial region, lesions located deeper required more energy to achieve the same volume of necrotic tissue [Citation16]. Our study shows that the abdominal wall thickness correlated well with NPV ratio, EEF, and greyscale change. For patients with thick abdominal walls, the EEF was significantly higher, the NPV ratio was significantly lower, and there was less greyscale change during HIFU treatment in comparison to patients with thin abdominal walls.
Meanwhile, this study also found that the NPV ratio was associated with the locations of adenomyosis and the uterus. The NPV ratio was significantly higher in patients with adenomyosis at the anterior wall of the anteverted uterus in comparison to the patients with adenomyosis at posterior wall of the retroverted uterus. We also found that significant greyscale change was associated with the location of the uterus, with the changes more likely to be seen with anteverted uteri. This can be explained by the shorter distance the ultrasound had to penetrate in the acoustic pathway of patients with anteverted uteri or the adenomyotic lesions being located at the anterior wall of the uteri, rather than that of patients with retroverted uteri or the lesions located at the posterior wall of the uterus. The attenuation of the ultrasound beam was less when the ultrasonic beam travelled through the shorter acoustic pathway, with more acoustic energy reaching the focal points. Since the main target was relatively further away from the sacrum, patients did not often complain of sciatic or buttock pain, and these patients tolerated the treatment well, which helped improve the efficiency of HIFU ablation.
During HIFU treatment, the ultrasound beams penetrate several layers of different tissue types in the acoustic pathway. Our study showed that EEF was positively related to the distance from the adenomyotic lesion ventral side to the skin (other than the thickness of the target lesion, including a variety of tissues such as skin, subcutaneous fat, muscle, peritoneum, bladder, uterine muscle wall, for example). Since the interfaces in the acoustic pathway would cause absorption, reflection, and scattering of the ultrasound beams, attenuation of the ultrasound beams occurred.
In this study we also found that the volume of the adenomyotic lesion was positively correlated with the NPV ratio, while negatively correlated with EEF, which means that the NPV ratio was significantly elevated and the EEF was significantly reduced in patients with larger adenomyotic lesions. This may be explained by ‘damage–damage interference effects’ because the expansion of the necrotic area and the ascent of temperature on the focal point will dynamically influence the acoustic environment of surrounding focus tissue and contribute to the ultrasonic energy deposition [Citation17]. An in vitro experimental study demonstrated that the EEF mass was less than the EEF slice, while the EEF slice was less than the EEF fascicle, which also could be used to explain this result [Citation18].
The blood supply of the adenomyotic lesions is related to the efficiency of HIFU ablation. The degree of enhancement clearly shows the blood supply of the adenomyotic lesions. Significant enhancement in the arterial phase usually shows rich blood supply. Our results suggest that hyper-enhancing lesions were difficult to treat, as the NPV ratio was low in this group. We further analysed the data and found that the EEF for the significantly enhanced lesions was significantly higher than that of the lesions with mild enhancement. This is likely secondary to the arterial blood flow, carrying the thermal energy away from the lesions more quickly. Decreasing the blood supply of the adenomyotic lesions by administration of oxytocin may help to improve the therapeutic efficiency of HIFU [Citation6].
MRI images can reflect biological characteristics of lesions. Different signal intensities on T2WI represent different biological characteristics. Funaki et al. [Citation19] reported that hyperintensity on T2WI indicates angiogenesis, rich aqueous tissue or degeneration of fibroids. The blood flow may take away part of the ultrasonic energy, resulting in energy loss and affecting efficacy of HIFU. Hypointensity of the lesions on T2WI suggests lack of angiogenesis and moisture content; hard for ultrasound to penetrate, and easy to deposit energy. Therefore, EEF for lesions with hypointensity signal on T2WI was lower. The hyperintense point seen in the adenomyotic lesions is a feature of adenomyosis, which can be used as favourable diagnostic evidence for adenomyosis. Hyperintense points may represent ectopic endometrial tissue, endometrial cyst or haemorrhage on histology [Citation20]. In many lesions, the scattered small hyperintense points can be seen in both T1WI and T2WI. This study showed that the adenomyotic lesions with rich scattered hyperintense points were difficult to treat, The NPV ratio in patients with multiple hyperintense points on T2WI images was lower than that of patients with adenomyotic lesions with no or little scattered hyperintense points. Repeated bleeding in the adenomyotic lesions with the menstrual cycle may lead to the changes in adenomyotic lesion structure and thus help to deposit the energy.
Limitations of the study are that it is retrospective and it utilised two separate HIFU units. Although JC and JC 200 are similar, the use of two separate machines may cause biased results. Another potential biased limitation is that the treatment was performed by different doctors. In addition, in measuring the adenomyotic lesion ventral side distance to skin by MRI, patients were in a supine position, but during the treatment patients were in a prone position, which could have changed the distance a little. Future studies should be more standardised to minimise any possible interference.
Conclusions
In summary, our results showed that multiple factors have an influence on USgHIFU for adenomyosis. The therapeutic efficiency was found to be less in patients with greater abdominal thickness, distance of the ventral side of adenomyosis to the skin, degree of arterial enhancement, and amount of high T2 signal in a lesion. This information can be used to guide patient selection and future design to optimise treatment.
Acknowledgements
We thank Wendy Zhang, a native English speaker from the University of California at San Diego, who has done a thorough review and correction of the English of this paper.
Disclosure statement
This work was partially supported by the National Basic Research Programme of China (2011CB707900), The National Natural Science Fund by the Chinese National Science Foundation (81127901, 11274404, 11574039), the National Key Technology R&D Programme (grant no. 2011BAI14B01). L.Z. is a senior consultant to Chongqing Haifu Company. The other authors have no conflict of interest to declare. The authors alone are responsible for the content and writing of the paper.
References
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus – revisited. Am J Obstet Gynecol 1972;112:583–93.
- Owolabi TO, Strickler RC. Adenomyosis: a neglected diagnosis. Obstet Gynecol 1977;50:424–27.
- Seidman JD, Kjerulff KH. Pathologic findings from the Maryland Women’s Health Study: practice patterns in the diagnosis of adenomyosis. Int J Gynecol Pathol 1996;15:217–21.
- Parazzini F, Mais V, Cipriani S, Busacca M, Venturini P; GISE. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: results from a prospective multicentric study in Italy. Eur J Obstet Gynecol Reprod Biol 2009;143:103–6.
- Wood C. Surgical and medical treatment of adenomyosis. Hum Reprod Update 1998;4:323–36.
- Zhang X, Zou M, Zhang C, He J, Mao S, Wu Q, et al. Effects of oxytocin on high intensity focused ultrasound (HIFU) ablation of adenomysis: a prospective study. Eur J Radiol 2014;83:1607–11.
- Zhang X, Li K, Xie B, He M, He J, Zhang L. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet 2014;124:207–11.
- Yang Z, Cao YD, Hu LN, Wang ZB. Feasibility of laparoscopic high-intensity focused ultrasound treatment for patients with uterine localized adenomyosis. Fertil Steril 2009; 91:2338–43.
- Wang W, Wang Y, Tang J. Safety and efficacy of high intensity focused ultrasound ablation therapy for adenomyosis. Acad Radiol 2009;16:1416–23.
- Zhou M, Chen JY, Tang LD, Chen WZ, Wang ZB. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 2011;95:900–5.
- Sudderuddin S, Helbren E, Telesca M, Williamson R, Rockall A. MRI appearances of benign uterine disease. Clin Radiol 2014;69:1095–104.
- Reinhold C, Tafazoli F, Wang L. Imaging features of adenomyosis. Hum Reprod Update 1998;4:337–49.
- Xiong Y, Yue Y, Shui L, Orsi F, He J, Zhang L. Ultrasound guided high intensity focused ultrasound (USgHIFU) ablation for the treatment of patients with adenomyosis and prior abdominal surgical scars: a retrospective study. Int J Hyperthermia 2015;31:777–83.
- Fry FJ. Intense focused ultrasound in medicine. Some practical guiding physical principles from sound source to focal site in tissue. Eur Urol 1993;23:2–7.
- Baker KG, Robertson VJ, Duck FA. A review of therapeutic ultrasound: biophysical effects. Phys Ther 2001;81:1351–8.
- ter Haar G, Sinnett D, Rivens I. High intensity focused ultrasound – a surgical technique for the treatment of discrete liver tumours. Phys Med Biol 1989;34:1743–50.
- Chen L, ter Haar G, Hill CR. Influence of ablated tissue on the formation of high-intensity focused ultrasound lesions. Ultrasound Med Biol 1997;23:921–31.
- Li F, Wang Z, Du Y, Ma P, Bai J, Wu F, et al. Study on therapeutic dosimetry of HIFU ablation tissue. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2006;23:839–43.
- Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol 2007;196:184.e1–6.
- Reinhold C, McCarthy S, Bret PM, Mehio A, Atri M, Zakarian R, et al. Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology 1996;199:151–8.