Abstract
Objective The aim of this study was to analyse the significant variables for vaginal discharge after ultrasound-guided percutaneous microwave ablation (PMWA) therapy.
Materials and methods PMWA was performed on 117 patients with adenomyosis from October 2012 to July 2014. The presence or absence, colour, quantity and duration of vaginal discharge, which was different from pre-ablation, were recorded within 1 year after PMWA. Patients were categorised into G1 (n = 26, without vaginal discharge), G2 (n = 40, vaginal discharge lasting 1 to 19 days), and G3 (n = 51, vaginal discharge lasting ≥20 days) groups. The potentially correlative variables were analysed. Variables with significant correlations with vaginal discharge post-ablation were identified via binary logistic regression analysis.
Results The differences in adenomyosis type, pre-ablation uterine volume, total microwave ablation energy, total non-perfused volume (NPV) and minimum distance from the non-perfused lesion (NPL) margin to the endomyometrial junction (EMJ) among groups were statistically significant (p = 0.005, p = 0.000, p = 0.000, p = 0.005 and p = 0.000, respectively). Minimum distance from the NPL margin to the EMJ was the strongest predictor of vaginal discharge post-ablation with odds ratio (OR) 0.632, p = 0.018, 95% CI 0.432–0.923. Patients with diffuse adenomyosis were more likely to have prolonged vaginal discharge (≥20 days) post-ablation (OR 3.461, p = 0.000, 95% CI 1.759–7.536).
Conclusion The minimum distance from the NPL margin to the EMJ and adenomyosis type were significantly associated with vaginal discharge post-ablation.
Introduction
Adenomyosis is one of the most common benign diseases among women of reproductive age. The mean occurrence rate of adenomyosis at hysterectomy among women of reproductive age ranges from 20–30%; however, its reported prevalence reaches 70% depending on the diagnostic criteria, the characteristics of the analysed samples, and the investigator’s skills [Citation1–2]. Patients often complain of menorrhagia, dysmenorrhoea, pelvic mass, infertility, and pelvic pressure symptoms, which seriously influence their quality of life. Bird et al. [Citation3] defined adenomyosis as the presence of endometrial tissue within the myometrium, which leads to the enlargement of uterine volume and presents under microscopic examination as ectopic non-neoplastic endometrial glands and stroma surrounded by hypertrophic and hyperplastic smooth muscle cells. Uncertainty in defining the site and the extent of adenomyosis makes it difficult to determine the accuracy and feasibility of complete excision when conserving the uterus. As a result, hysterectomy has remained the most popular surgical procedure for adenomyosis, which leads to loss of fertility [Citation4].
After Zhang et al. first applied ultrasound-guided percutaneous microwave ablation (PMWA) to treat uterine fibroids [Citation5], Ma et al. then reported the safety and effectiveness of PMWA in treating diffuse adenomyosis [Citation6]. As a new conservative therapy, ultrasound-guided PMWA for uterine benign diseases is a minimally invasive, safe, and effective treatment with short hospitalisation times, rapid recovery times, and the absence of severe complications [Citation5–8]. The ablation range and shape of PMWA can be controlled and undertaken under real-time ultrasound guidance without serious complications [Citation6]. Based on our clinical experience, the most common side effects of PMWA are lower abdominal pain and vaginal discharge [Citation5]. Lower abdominal pain can be alleviated or eliminated on the day of, or following treatment [Citation5]. Some patients experience a prolonged period of vaginal discharge, which influences their quality of life and can lead to secondary infections. The aim of this retrospective study is to determine the independent variables associated with the presence and duration of post-ablation vaginal discharge via statistical analysis.
Materials and methods
Patients
From October 2012 to July 2014, 117 patients, diagnosed with adenomyosis by ultrasonography and enhanced magnetic resonance imaging (ceMRI) at the Chinese PLA General Hospital, were recruited. Patients were screened with routine microbiological testing for vaginosis and treated prior to PMWA. All of the study patients were diagnosed with adenomyosis on ultrasonography and ceMRI pre-ablation. The criteria of adenomyosis for this study via ultrasonography were (1) asymmetric thickness of the anterior and posterior walls, (2) obscure endometrial-myometrial junctions, (3) sub-endometrial myometrial striations, (4) myometrial cysts, and (5) heterogeneous echogenicity of the myometrium [Citation9]. The criteria of adenomyosis for this study via MRI were (1) a myometrial mass with indistinct margins of primarily low intensity, (2) local or diffuse widening of junctional zones on T2-weighted images, (3) junctional zone thicknesses >35 mm, and (4) small hypointense myometrial spots or uterine enlargement [Citation10]. All of the patients were treated by the same operator to avoid introducing inter-operator error. PMWA for the treatment of uterine benign diseases was approved by the Chinese PLA General Hospital Institutional Review Board and registered online (ratification no. 20100930-004, registration no. ChiCTR-TRC-10001119)
Inclusion criteria were 1) symptomatic adenomyosis patients diagnosed by ultrasonography and ceMRI who lacked uterine fibroids and refused hysterectomy and other treatment options, 2) patients received no other treatments such as uterine artery embolisation (UAE), or high-intensity focused ultrasound (HIFU), and PMWA was not combined with other treatments such as ethanol injection, and 3) patients volunteered to undergo PMWA therapy and signed the informed consent prior to treatment.
Exclusion criteria were 1) patients with uterine fibroids, 2) patients who had undergone an intrauterine cavity operation of dilatation and curettage within 1 month before PMWA, 3) patients who had undergone IUD removal within 1 month before PMWA, 4) patients with coagulation disorders, pelvic infections or other malignant tumours confirmed by pathological examination, 5) patients who were menstruating, pregnant, or lactating, and (6) patients with incomplete medical records or who were lost to follow-up.
Variables of interest
Patient general clinical information was collected and included age, body mass index (BMI), obstetric history (gestation times, parity times, abortion times), previous intrauterine treatment (e.g. presence/absence of a Mirena intrauterine system), haemoglobin level, adenomyosis type, dysmenorrhoea score assessed using a visual analogue scale (VAS), Symptom Severity Score (SSS), health-related quality of life (HRQL) score from the Uterine Fibroid Symptoms Quality Of Life (UFS-QOL) questionnaire [Citation11,Citation12], phase of menstrual cycle upon treatment (pre-ovulation or post-ovulation), gonadal hormonal levels, and endometrial thickness.
The recorded lesion characteristics and microwave ablation relative variables comprised uterine volume pre-ablation, rate of uterine volume shrinkage 3 days post-ablation, minimum distance from the non-perfused lesion (NPL) margin to the endomyometrial junction (EMJ), total microwave ablation energy, and total non-perfused volume (NPV) 3 days post-ablation.
Microwave ablation equipment and procedure
A microwave ablation tumour coagulator (KY-2000 MW; Kangyou Medical Instruments, Nanjing, China) was used for therapy with a frequency of 2450 MHz and capability of continuous and pulse MW emission modes. The antenna was 15 gauge in diameter and 18 cm in length. An internal water cycle cooling system was used to cool the needle shaft. The distance from the microwave emission aperture to the needle tip was 11 mm. We used the Siemens Sequoia 512 ultrasound system (Acuson, Mountain View, CA, USA), with a transducer frequency range of 2.5–4.5 MHz. The instrument was equipped with a puncture guidance device and a low mechanical index contrast-enhanced function. The contrast agent used was SonoVue (manufactured by Bracco, Italy). Before injection, 5 mL of physiological saline was injected into 4.98 mg of frozen dry powder; the saline and powder were then shaken and the constituents intermingled to form a milk-white suspension containing sulphur hexafluoride capsules wrapped by phospholipids. Then, 2.4 mL of contrast agent was rapidly injected via the elbow vein, followed by the rapid injection of 5 mL of physiological saline for washing.
Before ablation, general patient clinical information was recorded. ceMRI was undertaken within 1 week before ablation. Adenomyosis type, lesion range and endometrium thickness were recorded, and uterine body volume was calculated. Measurements were made of the supero-inferior diameter and maximal anteroposterior diameter perpendicular to the supero-inferior diameter on the sagittal section and of the maximal transversal diameter on the transverse section. Uterine body volume was calculated as 0.5233 × supero-inferior diameter × anteroposterior diameter × transversal diameter [Citation13]. The patients were placed in a supine position. The PMWA was performed under intravenous conscious sedation (induction with 1.0 mg midazolam, 0.05 ng fentanyl and 1.0–1.5 mg/kg propofol; maintenance with 0.4–1.2 mg/kg/h propofol) by the same physician (Jing Zhang). Subsequently, the antenna was inserted into the lesion under ultrasound guidance. Proper microwave ablation power and action time were selected according to the dose–effect relationship of microwave ablation [Citation14]. The echo change of and around the ablated area was monitored under real-time ultrasonography. The emission was discontinued when the hyperecho (calculated according to microbubbles generated during microwave emission and roughly delineating the thermal field edge) reached 0.3 cm to the serosa inferior margin or covered more than 1/2 of the lesion range [Citation9]. Preliminary evaluation of ablation was performed by contrast-enhanced ultrasound immediately, and a supplementary treatment was performed when necessary [Citation15]. The total insertion times, the power and ablation time of each insertion, the microwave ablation energy which was calculated by ablation power × ablation time were recorded.ceMRI was performed within 3 days post-ablation to further evaluate the ablation range [Citation16]. Using MRI imaging, measurements were taken of the uterine body volume post-ablation, the minimum distance from the NPL margin to the EMJ (), and the total non-perfused lesion volume. The NPV is equal to the ablated lesion volume [Citation17]. The supero-inferior diameter, maximal anteroposterior diameter perpendicular to the supero-inferior diameter on the sagittal section and the maximal transversal diameter on the transverse section of the NPL were measured. NPV was calculated as 0.5233 × supero-inferior diameter × anteroposterior diameter × transversal diameter [Citation13].
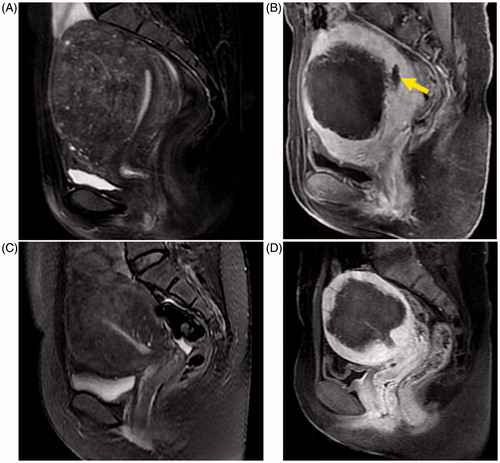
Figure 1. MRI images of the sagittal view in patients with adenomyosis. (A, C) T2-weighted images pre-ablation; globally enlarged uterus with local and diffuse widening of junctional zones can be seen in each image. (B, D) T1-weighted contrast-enhanced images showing non-perfused lesions corresponding to the ablated necrotic zone of A and C post-PMWA; the yellow arrow identifies the endometrium.
Data collection and patient groups
Follow-up examinations and phone calls every three months post-ablation were performed to record the presence or absence, colour, quantity, and duration of vaginal discharge, which was different from pre-ablation, within a 1-year period post-PMWA. For patients who complained of pruritus vulvae, malodour, burning micturition, or dysuria, routine microbiological testing was performed to exclude secondary infection [Citation18,Citation19]. We defined vaginal discharge lasting ≥20 days as prolonged vaginal discharge because about 75% of patients expected vaginal discharge lasting ≥20 days would influence their quality of life during follow-up. According to the presence/absence and duration of vaginal discharge, the patients were divided into groups: G1 (without vaginal discharge), G2 (vaginal discharge lasting 1–19 days), and G3 (vaginal discharge lasting ≥ 20 days).
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (IBM, Armonk, NY). Normally distributed continuous data were expressed as means ± SD, and non-normally distributed parameters were expressed as medians and inter-quartile ranges. For normally distributed continuous data, t-tests were performed for comparisons between two groups, and ANOVA tests were performed for comparisons among multiple groups. For non-normally distributed data, non-parametric Mann–Whitney U tests or Kruskal–Wallis tests were used. Fisher’s exact tests were performed when more than 20% of cells had expected counts of less than five. A binary logistic regression analysis was performed to test for the effects of the independent variables on vaginal discharge post-ablation. p-values of 0.05 or less were considered statistically significant.
Results
General conditions
The average patient age was 39.3 ± 4.9 years old (range 27–52 years old). The majority of the patients (77.8%) exhibited vaginal discharge to either a greater or lesser extent post-ablation. In some cases the discharge consisted of fluid without odour accompanied by small fragments of necrotic tissue of different sizes. The intermittent presence of necrotic tissue was reported by 15 G3 patients (29.4%) and two G2 patients (5%). The fragments of necrotic tissue ranged in size from 0.8 × 0.6 × 0.5 cm to 4.0 × 3.3 × 2.5 cm. Most of the patients complained of tolerable pain with a 2–3 VAS score and reported that the pain disappeared after the necrotic tissue was discharged. Only one patient suffered from pain with a VAS score of 4–5 during necrotic tissue discharge and self-administered oral relief medication as a result. In total, 14 patients that reported pruritus vulvae, malodour, burning micturition, or dysuria during follow-up were subsequently diagnosed with bacterial vaginosis. One patient suffered from bloody vaginal discharge with fetor and pelvic pain accompanied by low-grade fever 1 month post-PMWA. Multiple hyperechoic masses inside her uterine cavity were apparent on ultrasonography. The patient’s clinical history revealed that she had taken a hot-spring bath 1 month post-ablation. She was then hospitalised for endometritis and administered intravenous antibiotic therapy. She was discharged 1 week after treatment.
For most of the patients, vaginal discharge lasted from 0 to 60 days (mean ± SD 20.0 ± 26.4 days) and was predominantly characterised as pink, light red, yellow or brown. However, only one patient complained of vaginal discharge that lasted for more than 200 days and refused to come back for further diagnosis and treatment. According to the presence/absence and duration of vaginal discharge, the patients were divided into G1 (n = 26 patients, 22.2%), G2 (n = 40 patients, 34.2%) and G3 (n = 51, 43.6%) groups. Vaginal discharges that occurred within 20 days post-ablation were predominantly characterised as pink or light yellow. Among the G3 patients, the vaginal discharges over 20 days post-ablation were predominantly yellow or brown. The 14 patients diagnosed with vaginosis during the follow-up were all G3 patients. We hypothesise that the secondary infection prolonged the vaginal discharge post PMWA. The vaginal discharge was not directly caused by bacterial vaginosis, which is the most common cause of vaginal discharge among women [Citation20].
Patient baseline characteristics
The general clinical information of patients is provided in . No significant differences in age, BMI, obstetric history (gestation times, parity times, abortion times), previous intrauterine treatment, phase of menstrual cycle upon treatment, endometrial thickness, gonadal hormone levels, haemoglobin level, dysmenorrhoea score or SSS or HRQL score of the UFS-QOL questionnaire (p > 0.05) were observed among the groups. However, adenomyosis type differed significantly among the groups (p = 0.005).
Table 1. Patient baseline characteristics; means ± SD or M (P25∼P75).
The lesion characteristics and microwave ablation relative parameters are provided in . The inter-group differences in pre-ablation uterine volume and minimum distance from the NPL margin to the EMJ were significant (p = 0.000 and p = 0.000). There was no significant difference in the rate of uterine volume shrinkage among the groups (p = 0.503). The differences in total microwave ablation energy and total NPV among the groups were significantly different (p = 0.000 and p = 0.005, respectively).
Table 2. Comparison of characteristics of lesion & microwave ablation relative parameters among groups; means ± SD or mean (P25∼P75).
Logistic regression
Significantly correlative variables (adenomyosis type, uterine volume pre-ablation, minimum distance of the NPL margin to the EMJ, total ablation energy, and total NPV 3 days post-ablation) were included in the logistic regression model. Minimum distance from the NPL margin to the EMJ had the strongest effect on vaginal discharge post-ablation (OR 0.632, p = 0.018, 95% CI 0.432–0.923) (). For the group without discharge fluid post-ablation, the average distance from the NPL margin to the EMJ was 8 mm (range 2.8–20 mm).
Table 3. Logistic regression analysis of variables associated with presence of vaginal discharge post-ablation.
The average duration of vaginal discharge post-ablation was 20 days. For the group with a vaginal discharge duration post-ablation of ≥20 days, the independent variables with significant correlation included adenomyosis type (OR 3.461, p = 0.000, 95% CI 1.759–7.536) and minimum distance from the NPL margin to the EMJ (OR 0.003, p = 0.000, 95% CI 0.000–0.054) when compared to the group with vaginal discharge post-ablation lasting approximately 1–19 days (). Adenomyosis type and minimum distance from the NPL margin to the EMJ were significant predictors of the duration of vaginal discharge post-ablation.
Table 4. Logistic regression analysis of variables associated with prolonged (≥ 20 days) vaginal discharge post-ablation.
Discussion
To date, there has been no consensus on the most appropriate treatment for infertile patients with adenomyosis [Citation21]. Hormonal therapy [Citation22], uterine arterial embolisation (UAE) [Citation23] and conservative surgical procedures [Citation24] have been used to treat infertile patients with adenomyosis. Ma et al. [Citation6,Citation10] first reported the effectiveness of PMWA in treating diffuse adenomyosis; PMWA resulted in the elimination or alleviation of symptoms associated with this condition. Yang et al. [Citation10] showed that there was no influence of PMWA treatment on ovarian function. There is no discernible delineation between an adenomyosis lesion and normal tissue that could be used to localise thermal energy except in the case of uterine fibroids. Therefore, PMWA treatment for adenomyosis may damage the endometrium more easily than for uterine fibroids. In the present study some patients complained of prolonged vaginal discharge post-ablation, which can influence patient quality of life and potentially lead to secondary infections.
The type of denomyosis, uterine volume pre-ablation, the minimum distance from the NPL margin to the EMJ, total ablation energy, and total NPV 3 days post-ablation were significantly associated with vaginal discharge post-ablation. At short minimum distances from the NPL margin to the EMJ, the endometrium can be easily damaged because the adenomyosis lesion has no well-defined boundary separating it from normal tissue. Thermal damage to the endometrium may lead to vaginal discharge post-ablation. The results of the present study suggest that the occurrence rate of vaginal discharge post-ablation significantly decreases at minimum distances from the NPL margin to the EMJ > 8 mm. In the group with a prolonged duration of vaginal discharge post-ablation (>20 days), adenomyosis type, and the minimum distance from the NPL margin to the EMJ had significant effects on the duration of vaginal discharge. The greater the uterine volume pre-ablation was, the greater were both the lesion range and the total NPV 3 days post-ablation. The logistic regression analysis showed that the aforementioned parameters had no significant effects on the duration of vaginal discharge post-ablation. Moreover, the difference in uterine volume pre-ablation between the diffuse and focal adenomyosis patients was significant. The uterine volume of diffuse adenomyosis patients (337.63 ± 168.92 cm3) was greater than that of focal adenomyosis patients (275.79 ± 160.72) (p = 0.049). This result may explain why uterine volume pre-ablation, total ablation energy, and total NPV had no significant effects on the duration of vaginal discharge post-ablation: all three variables may be correlated with adenomyosis type. The differences in uterine volume pre-ablation (p = 0.001), total ablation energy (p = 0.011), and total NPV (p = 0.009) between the G2 and G3 groups were significant, and these variables were strongly associated with the duration of vaginal discharge post-ablation. Although these variables did not have significant effects on the duration of vaginal discharge, they were strongly correlated with each other. Clinical examinations performed during the follow-up period revealed a zone of liquidation necrosis () within the ablation area during ultrasonography in a subset of patients with relatively prolonged vaginal fluid discharge, which strongly suggests that relatively prolonged vaginal discharge may be the result of tissue with liquidation necrosis via the passage to the endometrium.
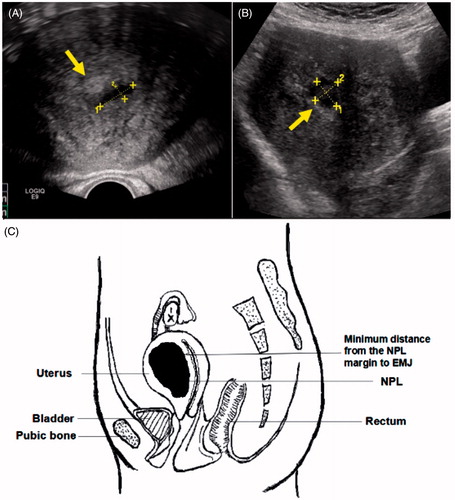
Figure 2. (A, B) The zone of liquidation necrosis as observed via ultrasonography; yellow arrows identify the endometrium. The liquidation necrotic zones were connected to the endometrium. (C) Schematic diagram post-ablation. EMJ, endomyometrial junction; NPV, non-perfused lesion volume; NPL, non-perfused lesion.
This was a retrospective study in which influential variables on vaginal discharge after PMWA were identified via statistical analysis. However, future research is required to identify the pathogenic mechanisms underlying this process (e.g. the occurrence and degree of any permanent damage to the endometrium, and the pathological process of endometrial recovery). We hope that this study will aid clinicians in alleviating or avoiding thermal damage to the endometrium when performing PMWA therapy and other thermal ablation therapies in situ.
In conclusion, the minimum distance from the NPL margin to the EMJ was the strongest predictor of the presence of vaginal discharge post -MWA treatment. Compared with focal adenomyosis patients, patients with diffuse adenomyosis are at a higher risk of developing relatively prolonged vaginal discharge post-ablation.
Disclosure statement
This study was supported by the National Natural Science Foundation of China (grant no. 81371562). There are no conflicts of interest reported by authors. The authors alone are responsible for the content and writing of the paper.
References
- Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol 2016;23:164–85.
- Graziano A, Lo Monte G, Piva I, Caserta D, Karner M, Engl B, et al. Diagnostic findings in adenomyosis: a pictorial review on the major concerns. Eur Rev Med Pharmacol Sci 2015;19:1146–54.
- Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus revisited. Am J Obstet Gynecol 1972;112:583–93.
- Wood C. Adenomyosis: difficult to diagnose, and difficult to treat. Diagn Ther Endosc 2001;7:89–95.
- Zhang J, Feng L, Zhang BS, Ren J, Li Z, Hu D, et al. Ultrasound-guided percutaneous microwave ablation for symptomatic uterine fibroid treatment – a clinical study. Int J Hyperthermia 2011;27:510–16.
- Ma X, Zhang J, Han ZY, Cai JM, Zhou HY, Xu RF, et al. Feasibility study on energy prediction of microwave ablation upon uterine adenomyosis and leiomyomas by MRI. Br J Radiol 2014;87:20130770. doi: 10.1259/bjr.20130770. Epub 2014 Jun 20.
- Ma X, Zhang J, Han ZY, Yang Y, Hao YL, Xu CT, et al. Research of dose–effect relationship parameters of percutaneous microwave ablation for uterine leiomyomas – a quantitative study. Sci Rep 2014;4:6469. doi: 10.1038/srep06469.
- Wang F, Zhang J, Han ZY, Cheng ZG, Zhou HY, Feng L, et al. Imaging manifestation of conventional and contrast-enhanced ultrasonography in percutaneous microwave ablation for the treatment of uterine fibroids. Eur J Radiol 2012;81:2947–52.
- Maheshwari, A., Gurunathi, S., Fatima, F., Bhattacharya, S. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update 2012.;18:374–92.
- Yang Y, Zhang J, Han ZY, Ma X, Hao YL, Xu CT, et al. Ultrasound-guided percutaneous microwave ablation for adenomyosis: efficacy of treatment and effect on ovarian function. Sci Rep 2015;5:10034. doi: 10.1038/srep10034.
- Zhang X, Li K, Xie B, He M, He J, Zhang L. Effective ablation therapy of adenomyosis with ultrasound-guided high-intensity focused ultrasound. Int J Gynaecol Obstet 2014;124:207–11.
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz MK, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300.
- Ruhnke H, Eckey T, Bohlmann MK, Beldoch MP, Neumann A, Agic A, et al. MR-guided HIFU treatment of symptomatic uterine fibroids using novel feedback-regulated volumetric ablation: effectiveness and clinical practice. Rofo 2013;185:983–91.
- Zhou W, Liang M, Pan H, Liu X, Jiang Y, Wang Y, et al. Comparison of ablation zones among different tissues using 2450-MHz cooled-shaft microwave antenna: results in ex vivo porcine models. PLoS One 2013;8:71873. doi: 10.1371/journal.pone.0071873.eCollection 2013
- Lei F, Jing Z, Bo W, Dongme H, Zhencai L, Xue J, et al. Uterine myomas treated with microwave ablation: the agreement between ablation volumes obtained from contrast-enhanced sonography and enhanced MRI. Int J Hyperthermia 2014;30:11–18.
- Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 2013;19:5430–8.
- Kroencke T J, Scheurig C, Kluner C, Taupitz M, Schnorr J, Hamm B. Uterine fibroids: contrast-enhanced MR angiography to predict ovarian artery supply – initial experience. Radiology 2006;241:181–9.
- Baruah FK, Sharma A, Das C, Hazarika NK, Hussain JH. Role of Gardnerella vaginalis as an etiological agent of bacterial vaginosis. Iran J Microbiol 2014;6:409–14.
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach DA, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14–22.
- Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301.
- Wang PH, Yang TS, Lee WL, Chao HT, Chang SP, Yuan CC. Treatment of infertile women with adenomyosis with a conservative microsurgical technique and a gonadotropin-releasing hormone agonist. Fertil Steril 2000;73:1061–2.
- Silva PD, Perkins HE, Schauberger CW. Live birth after treatment of severe adenomyosis with a gonadotropin-releasing hormone agonist. Fertil Steril 1994;61:171–2.
- Agdi M, Valenti D, Tulandi T. Intraabdominal adhesions after uterine artery embolization. Am J Obstet Gynecol 2008;199:482.e1–3. doi: 10.1016/j.ajog.2008.04.006. Epub 2008 May 19.
- Takeuchi H, Kitade M, Kikuchi I, Shimanuki H, Kumakiri J, Kitano T, et al. Laparoscopic adenomyomectomy and hysteroplasty: a novel method. J Minim Invasive Gynecol 2006;13:150–4.
