Abstract
Background Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) is a promising approach for preventing peritoneal carcinomatosis in high-risk patients. We report our initial experience with prophylactic HIPEC in a series of patients with appendiceal neoplasms. Methods We retrospectively reviewed our prospectively maintained database to identify patients who underwent HIPEC in the absence of peritoneal disease. Patients with previously documented peritoneal surface disease were excluded. Data regarding clinical, operative and pathological features were analysed. Results Out of 322 HIPEC procedures performed between March 2007and August 2015, we identified 16 patients who underwent surgery with prophylactic intent. Primary diagnoses included high-grade and low-grade appendiceal neoplasms. Most patients presented originally with appendiceal perforation; all patients underwent initial surgery during which the appendix or right colon were resected. Following a median time interval of 2.2 months, a second surgery performed at our institution consisted of completion of omentectomy, partial colectomy and oophorectomy, with administration of prophylactic HIPEC (using mitomycin C). A totally laparoscopic approach was attempted and achieved in 11 patients in whom the median duration of surgery, estimated intraoperative blood loss and length of hospitalisation were 251 min, 100 cm3 and 4 days, respectively. There were no cases of major perioperative morbidity or mortality. Conclusions Prophylactic HIPEC for appendiceal neoplasms is feasible, safe and may be performed laparoscopically. Larger studies with long-term follow-up are needed to determine whether a survival benefit is associated with this treatment.
Introduction
Peritoneal spread of any primary cancer has been historically regarded as a grim prognostic factor with limited curative potential. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC), pioneered in the 1980s by Sugarbaker [Citation1], is gaining acceptance as the standard of care treatment for peritoneal carcinomatosis of colorectal and appendiceal origin in selected cases [Citation2]. Historically, this treatment was reserved for advanced patients with significant carcinomatosis; however, over the past decade there has been a distinct shift towards earlier use of CRS/HIPEC based on the finding of a disease-burden dependent success rate [Citation3] with better outcomes observed in low tumour volume. Given the improved outcomes with lesser disease, several centres have investigated the impact of prophylactic HIPEC procedures in patients with gastric [Citation4,Citation5] and colorectal [Citation6–8] malignancies at high risk of peritoneal spread. However, there are fewer data available on prophylactic HIPEC in patients with appendiceal neoplasms, which frequently present with perforation.
Currently, no guidelines exist for the treatment of perforated epithelial appendiceal neoplasms. It is well established that low-grade appendiceal mucinous neoplasms (LAMN) confined to the appendix do not require any further treatment beyond an appendectomy [Citation9,Citation10]. In cases of appendiceal neoplasms with overt peritoneal dissemination, therapeutic CRS/HIPEC has shown promising results [Citation11,Citation12]. In between these two extremes, cases of perforated appendiceal neoplasm without apparent peritoneal spread, pose a therapeutic dilemma with treatment options ranging from expectant observation [Citation13] to prophylactic HIPEC [Citation14,Citation15]. We report our initial experience with prophylactic HIPEC in a series of 16 patients with epithelial appendiceal neoplasms, most of which presented as perforated tumours. In addition, we describe the feasibility of laparoscopic prophylactic HIPEC performed in a subgroup of patients.
Methods
After obtaining Institutional Review Board approval, we retrospectively reviewed our prospectively maintained database to identify patients who underwent HIPEC procedures with clear prophylactic intent. Indications for prophylactic HIPEC included perforated or invasive (pT3/4NxM0) epithelial appendiceal neoplasms resected previously (). HIPEC procedures performed in patients with previously documented peritoneal surface disease, including those who underwent preceding peritoneal debulking and those who had complete response to systemic chemotherapy, were not considered prophylactic and were excluded from our cohort. Data regarding clinical, operative and pathological features were analysed.
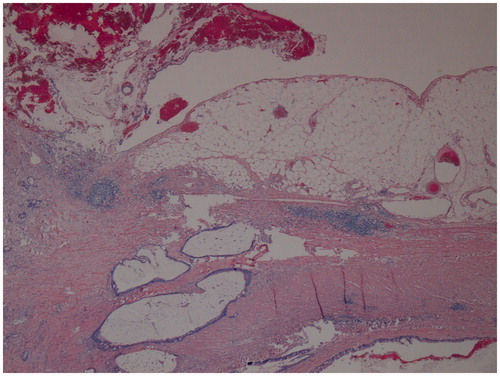
Figure 1. Pathological examination from the initial appendectomy performed in a 60-year-old woman, demonstrating mucinous adenocarcinoma with invasion of the appendiceal wall by gland-forming malignant cells and dissecting mucin (haematoxylin and eosin, original magnification ×40).
Our preoperative assessment included review of original pathology slides, review of cross-sectional imaging of the chest, abdomen and pelvis and a multidisciplinary conference discussing each individual case. From March 2007 to March 2010, all HIPEC procedures commenced routinely with exploratory laparotomy. Beginning on April 2010, as diagnostic laparoscopy was incorporated into our surgical practice [Citation16], a totally laparoscopic approach was attempted in prophylactic cases. During exploration of the peritoneal cavity, frozen section biopsies of any suspicious lesions were taken. Prophylactic omentectomy was performed routinely and the right colon was resected according to oncological principles (if not previously resected) in cases of high-grade epithelial appendiceal neoplasms. Prophylactic HIPEC was then delivered via the closed abdomen technique, using mitomycin C which was administered over two doses for a 90-min perfusion period with a target intraperitoneal temperature of 41–43 °C. A 40-mg dose was used and split between 30 mg for the first 60 min and 10 mg for the last 30 min, in accordance with consensus guidelines [Citation17]. Following the administration of HIPEC, gastrointestinal anastomoses were created when applicable.
Major post-operative morbidity was defined as Clavien-Dindo classification III–V and included any complication requiring invasive intervention, life-threatening conditions requiring intensive care unit management, or death [Citation18]. After hospital discharge, patients were followed every 6 months by physical exam and contrast-enhanced CT scan (or MRI) of the chest, abdomen and pelvis. Carcinoembryonic antigen levels were routinely used as part of surveillance in patients who demonstrated elevated levels at initial diagnosis.
Results
Out of 322 HIPEC procedures performed between March 2007 and August 2015, we identified 16 patients with appendiceal neoplasms who underwent surgery with prophylactic intent. Primary diagnoses were mucinous appendiceal adenocarcinoma (n = 7), goblet cell carcinoid (GCC) (n = 4) and LAMN (n = 5). Baseline patient characteristics are presented in . Almost all (n = 15) patients presented originally with signs and symptoms of acute appendicitis () and underwent initial emergent surgery during which the appendix or right colon was resected; of those, 13 patients had a perforated appendix at presentation. The remaining patient underwent semi-elective appendectomy, after a computed tomography scan performed for investigation of weight loss incidentally demonstrated dilated appendix. All but two of the cases of primary appendectomy/colectomy were performed at outside institutions and patients were referred in the early post-operative period to our institution for further treatment recommendations. None of the patients received systemic chemotherapy after the initial surgery. Following a median time interval of 2.2 (range 0.8–15.4) months, a second surgery performed at our institution consisted of completion of omentectomy (n = 14), right colectomy (n = 7) and oophorectomy (n = 2), with administration of prophylactic HIPEC. None of the patients had evidence of peritoneal carcinomatosis or mucinosis, either preoperatively or intraoperatively; in 13 out of 16 cases frozen section biopsies were taken from suspected peritoneal sites, and yielded benign findings. A totally laparoscopic approach () was attempted and achieved in 11 patients (69%); the remaining 5 patients were selected for laparotomy upfront: 3 patients underwent surgery before April 2010, when diagnostic laparoscopy was incorporated into our treatment algorithm; 2 patients were operated on after April 2010 but preferred the open approach over laparoscopy. There were no documented cases of failed laparoscopy. Intraoperative and post-operative patient characteristics are presented in . There were no cases of major perioperative morbidity or mortality. Four patients developed minor complications such as abdominal pain, wound infection, lung atelectasis or transient neutropenia. The median follow-up period was 12.2 months (range 2.2–67.4) from the date of diagnosis (range 8.8 months (1–65) from the date of prophylactic HIPEC), during which one patient with appendiceal adenocarcinoma who originally presented with a perforated appendix developed disease recurrence 9 months following prophylactic HIPEC. Two patients with appendiceal adenocarcinoma received adjuvant systemic chemotherapy (12 cycles of the FOLFOX regimen) after prophylactic HIPEC; the reasoning for chemotherapy was lymph node-positive disease in one case and moderate-poor differentiation with signet ring cells in the other case.
Figure 2. A computed tomography scan (coronal view) of a 63-year-old man who presented with signs and symptoms of acute appendicitis. The scan showed distended appendix (arrow). The patient underwent emergent appendectomy and pathological exam diagnosed mucinous appendiceal adenocarcinoma.
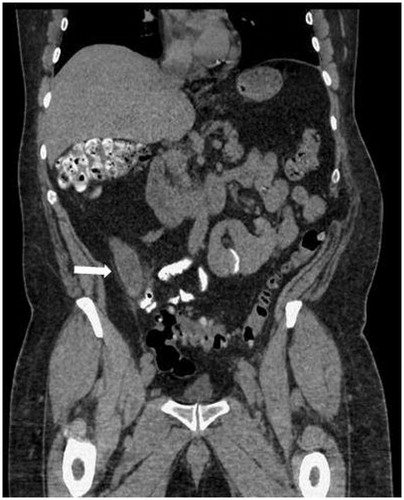
Figure 3. Laparoscopic administration of prophylactic hyperthermic intraperitoneal chemotherapy. The periumbilical incision was used for extraction of resected omentum/colon.
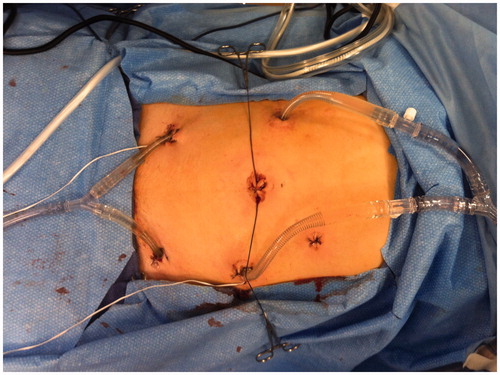
Table 1. Baseline characteristics of 16 patients with epithelial appendiceal neoplasms who underwent prophylactic HIPEC procedures.
Table 2. Intraoperative and postoperative characteristics of patients with appendiceal neoplasms who underwent prophylactic HIPEC procedures.
Discussion
Theoretically, tumour perforation or penetration through the appendiceal wall may result in seeding of isolated neoplastic cells throughout the peritoneal cavity. This early cancer stage, if left untreated, may eventually lead to the development of pseudomyxoma peritonei (PMP) – a progressive, lethal condition. The rationale for prophylactic HIPEC is to expose those isolated tumour cells to high regional concentrations of chemotherapy at an early stage most amenable to intervention [Citation6]. However, when considering prophylactic HIPEC, the morbidity associated with this procedure must be weighed against the potential oncological benefit and the probability of disease progression.
The risk of developing peritoneal carcinomatosis in cases of perforated mucinous appendiceal tumours is notoriously difficult to predict, since most available literature is focused on patients with tumours limited to the appendix or with widespread peritoneal disease. The largest series that addressed the prognostic significance of appendiceal perforation with localised extra-appendiceal mucin was published by Yantiss et al. [Citation19]: multi-institutional data from 65 patients with mucinous appendiceal neoplasms were analysed; after a median follow-up of 52 months, only two patients (4%) with acellular extra-appendiceal mucin developed diffuse peritoneal disease, whereas five out of 15 (33%) patients with cellular extra-appendiceal mucin developed PMP 24–87 months following appendectomy. Disease progression was also associated with high-grade histology. In another similar study by Pai et al. [Citation10], one out of 14 patients with perforated LAMN and extra-appendiceal acellular mucin developed PMP after 45 months. In contrast, patients with extra-appendiceal neoplastic epithelium (n = 27) and those with high-grade/invasive histology (n = 9) achieved lower 5-year disease-free survival rates – 25% and 20%, respectively. A population-based study from the Netherlands found that 20% of patients with mucinous epithelial neoplasm of the appendix ultimately developed PMP; in addition, out of 130 patients who presented with signs of a ruptured appendix, 21 patients (16%) were eventually diagnosed with PMP – most of whom were patients with mucinous cystadenoma or mucinous adenocarcinoma [Citation20].
Regarding perforated goblet cell carcinoid tumours, available literature is even scarcer. A systematic literature review by Madani et al. [Citation21] identified 78 GCC patients from seven articles, among which 18 perforations occurred: of these, metastatic spread to the peritoneum was described in one case and two tumour-related deaths occurred; however, peritoneal metastasis was also reported in non-perforated cases. Therefore, the authors concluded that peritoneal carcinomatosis is much more common in GCC as opposed to classical appendiceal carcinoids, but the prognostic impact of perforation remains unclear. In a recently published study from the Memorial Sloan Kettering Cancer Center, New York, that consisted of 70 patients with localised (pTxNxM0) high-grade appendiceal neoplasms (including 38 GCC patients), lymph node-positive disease at the time of initial surgery was found to be the only predictor of disease recurrence. Appendiceal perforation at initial presentation was more common among patients that eventually developed recurrence, but this difference was statistically insignificant [Citation22].
The long-term outcomes following prophylactic HIPEC for high-grade appendiceal neoplasms are still unknown. Prophylactic HIPEC for LAMN has been reported previously by McDonald et al. [Citation15]: 17 patients with histological diagnosis of type II LAMN (defined as mucin and/or neoplastic epithelium in the appendiceal submucosa, wall, and/or periappendicular tissue, with or without perforation) underwent risk-reducing CRS/HIPEC; after a median follow-up of 40 months, no cases of disease progression were observed. However, not all procedures were prophylactic in nature, since small-volume residual mucinous disease was noted during surgery in seven patients. This group from Manchester was also the first to report laparoscopic delivery of prophylactic HIPEC in patients with appendiceal neoplasms [Citation23]: 10 patients with type II LAMN were treated by minimal access cytoreductive surgery and HIPEC (n = 3 hand-port-assisted surgery; n = 7 totally laparoscopic); there were no conversions to laparotomy, and morbidity was comparable to open procedures. The use of laparoscopy in patients with peritoneal carcinomatosis has been described previously, but mainly in the context of other indications such as initial diagnosis of peritoneal disease, assessment of tumour burden in order to exclude patients from incomplete cytoreduction, cytoreduction of low volume disease and delivery of palliative HIPEC [Citation16,Citation24,Citation25].
Our data shows that laparoscopic prophylactic HIPEC is feasible and safe. There were no documented cases of major morbidity, mortality or conversion to laparotomy in our group of patients. To the best of our knowledge, our study, which represents one of the largest series describing laparoscopic administration of prophylactic HIPEC, is the first to report its use in high-grade appendiceal tumours. We advocate the use of prophylactic HIPEC in patients with perforated epithelial appendiceal neoplasms (including cases of LAMN with acellular periappendicular mucin) or high-risk high-grade appendiceal tumours (pT3/4N0M0 or positive lymph node status). We feel that the above-mentioned rates of disease progression in untreated patients justify this approach, especially in young patients with long life expectancy or patients requiring additional oncological surgery such as completion of right colectomy. In the latter case, the additive morbidity of HIPEC is even lower.
Currently, there is not enough evidence to support the use of systemic chemotherapy in cases of perforated/localised epithelial appendiceal neoplasms, although a recent large study by Asare et al. [Citation26] has shown that adjuvant chemotherapy improved overall survival in patients with localised appendiceal adenocarcinoma (stage I to III) for both mucinous and non-mucinous histologies; interestingly, patients with well-differentiated mucinous metastatic appendiceal adenocarcinomas derived no survival benefit from systemic chemotherapy. Other studies have reported that systemic chemotherapy may benefit patients with unresectable or metastatic high-grade disease [Citation27]. There is no clinical evidence justifying the use of systemic chemotherapy for LAMN [Citation27].
In conclusion, while there is growing evidence suggesting positive therapeutic effect of optimal CRS/HIPEC in appendiceal cancers with peritoneal spread, limited data are available regarding the prophylactic effect of HIPEC. Our preliminary study demonstrates that prophylactic HIPEC for epithelial appendiceal neoplasms is feasible, safe and may often be performed laparoscopically with acceptable morbidity and no mortality. The follow-up period for this series is relatively short as the median interval between diagnosis of primary appendiceal tumours and diagnosis of metachronous PMP has been reported as 2 years [Citation20]. Further, larger studies with long-term follow-up are needed to determine whether a survival benefit is associated with this treatment. Given the rare incidence of appendiceal epithelial lesions (approximately nine cases per million per year [Citation20]), multi-institutional collaboration is essential for addressing this question.
Acknowledgements
This paper was first presented at the 10th International Symposium on Regional Cancer Therapies meeting in Clearwater, FL, USA, 14–16 February 2015.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir 1988;373:189–96.
- Esquivel J, Elias D, Baratti D, Kusamura S, Deraco M. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008;98:263–7.
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43.
- Saladino E, Fleres F, Mazzeo C, Pruiti V, Scollica M, Rossitto M, et al. The role of prophylactic hyperthermic intraperitoneal chemotherapy in the management of serosal involved gastric cancer. Anticancer Res 2014;34:2019–22.
- Graziosi L, Cantarella F, Mingrone E, Gunnellini M, Cavazzoni E, Liberati M et al. Preliminary results of prophylactic HIPEC in patients with locally advanced gastric cancer. Ann Ital Chir 2013;84:551–6.
- Sugarbaker PH. Update on the prevention of local recurrence and peritoneal metastases in patients with colorectal cancer. World J Gastroenterol 2014;20:9286–91.
- Baratti D, Kusamura S, Deraco M. Colorectal cancer peritoneal metastases: second-look laparotomy, prophylactic HIPEC, or both? Ann Surg 2014;263:e5.
- Razenberg LG, van Gestel YR, Creemers GJ, Verwaal VJ, Lemmens VE, de Hingh IH. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol 2015;41:466–71.
- Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol 2003;27:1089–103.
- Pai RK, Beck AH, Norton JA, Longacre TA. Appendiceal mucinous neoplasms: clinicopathologic study of 116 cases with analysis of factors predicting recurrence. Am J Surg Pathol 2009;33:1425–39.
- Votanopoulos KI, Russell G, Randle RW, Shen P, Stewart JH, Levine EA. Peritoneal surface disease (PSD) from appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): overview of 481 cases. Ann Surg Oncol 2015;22:1274–9.
- Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449–56.
- Zih FS, Wong-Chong N, Hummel C, Petronis J, Panzarella T, Pollett A, et al. Mucinous tumor of the appendix with limited peritoneal spread: is there a role for expectant observation? Ann Surg Oncol 2014;21:225–31.
- Dhage-Ivatury S, Sugarbaker PH. Update on the surgical approach to mucocele of the appendix. J Am Coll Surg 2006;202:680–4.
- McDonald JR, O’Dwyer ST, Rout S, Chakrabarty B, Sikand K, Fulford PE, et al. Classification of and cytoreductive surgery for low-grade appendiceal mucinous neoplasms. Br J Surg 2012;99:987–92.
- Tabrizian P, Jayakrishnan TT, Zacharias A, Aycart S, Johnston FM, Sarpel U, et al. Incorporation of diagnostic laparoscopy in the management algorithm for patients with peritoneal metastases: a multi-institutional analysis. J Surg Oncol 2015;111:1035–40.
- Turaga K, Levine E, Barone R, Sticca R, Petrelli N, Lambert L, et al. Consensus guidelines from the American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol 2014;21:1501–5.
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009; 250:187–96.
- Yantiss RK, Shia J, Klimstra DS, Hahn HP, Odze RD, Misdraji J. Prognostic significance of localized extra-appendiceal mucin deposition in appendiceal mucinous neoplasms. Am J Surg Pathol 2009;33:248–55.
- Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 2008;34:196–201.
- Madani A, van der Bilt JD, Consten EC, Vriens MR, Borel Rinkes IH. Perforation in appendiceal well-differentiated carcinoid and goblet cell tumors: impact on prognosis? A systematic review. Ann Surg Oncol 2015;22:959–65.
- Nash GM, Smith JD, Cercek A, Tang L, Weiser MR, Temple LK, et al. Lymph node metastasis predicts disease recurrence in a single-center experience of 70 stages 1–3 appendix cancers: a retrospective review. Ann Surg Oncol 2015;22:3613–17.
- Fish R, Selvasekar C, Crichton P, Wilson M, Fulford P, Renehan A, et al. Risk-reducing laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasm: early outcomes and technique. Surg Endosc 2014;28:341–5.
- Esquivel J, Averbach A, Chua TC. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with limited peritoneal surface malignancies: feasibility, morbidity and outcome in an early experience. Ann Surg 2011;253:764–8.
- Sommariva A, Zagonel V, Rossi CR. The role of laparoscopy in peritoneal surface malignancies selected for hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2012;19:3737–44.
- Asare EA, Compton CC, Hanna NN, Kosinski LA, Washington MK, Kakar S, et al. The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer 2015;122:213–21.
- Barrios P, Losa F, Gonzalez-Moreno S, Rojo A, Gómez-Portilla A, Bretcha-Boix P, et al. Recommendations in the management of epithelial appendiceal neoplasms and peritoneal dissemination from mucinous tumours (pseudomyxoma peritonei). Clin Transl Oncol 2015. PMID 26489426.
