Abstract
Background This paper reports a study of 21 patients with peritoneal carcinomatosis from ovarian cancer who underwent cytoreductive surgery and HIPEC by means of PRS-1.0 Combat®, a new model for closed abdomen HIPEC aimed at improving fluid distribution with assistance from a CO2 recirculation system. This new technology has been previously shown to be successful in an experimental study (pig model) performed by our group, and has been approved for use in our hospital. Methods Twenty-one patients with peritoneal carcinomatosis of ovarian cancer origin were included in the study. Cytoreductive surgery and HIPEC were performed by a closed abdomen fluid and CO2 recirculation technique using the PRS-1.0 Combat® model. We analysed the intraoperative safety tolerance and post-operative morbidity and mortality during the first 30 days. Results Between November 2011 and March 2014 21 patients with epithelial ovarian cancer, International Federation of Gynecology and Obstetrics stage II–IV, were included in the study. During the procedure there were no significant haemodynamic or analytical disturbances. Complication rates were 38.1% and 57.14% for grade III/IV and minor (grade I/II) complications, respectively. Post-operative mortality was 4.76% (one patient). Complete cytoreductive surgery and intraperitoneal chemotherapy improved overall survival and disease-free survival in women with advanced ovarian cancer. The association of intra-abdominal hyperthermia with chemotherapy (HIPEC) increased the therapeutic benefit. Conclusions This study has shown that closed abdomen intraperitoneal chemohyperthermia by a fluid and CO2 recirculation system (PRS-1.0 Combat®) can be a safe and feasible model for the treatment of peritoneal carcinomatosis of ovarian cancer origin.
Keywords:
Introduction
The development and improvement of locoregional treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal chemotherapy has increased overall survival and disease-free survival for some tumour types, such as ovarian cancer or colon cancer [Citation1]. Debulking techniques involve the surgical removal of all macroscopically visible tumour volume by visceral resections and peritonectomy. Intraperitoneal chemotherapy acts on microscopic residual disease with an enhanced intracavitary therapeutic dose, leading to less toxicity than an intravenous dose. The hyperthermic drug solution increases tumour apoptosis by its direct cytotoxic effect and improves chemotherapeutic toxicity against tumour cells [Citation2,Citation3].
The standard technique for intraperitoneal hyperthermia chemotherapy (HIPEC) is an open abdomen technique, or Coliseum technique, developed by Sugarbaker for the treatment of patients with peritoneal carcinomatosis. However, modifications to this approach have recently been developed to better control the hyperthermia and reduce the risk of contamination of medical personnel (e.g. semi-closed or closed techniques) [Citation4].
In collaboration with industry, we have developed a model for closed abdomen HIPEC, with recirculation of CO2 and fluids (PRS-1.0 Combat®). The safety and effectiveness of this new technology was previously tested in an animal model [Citation5]. Our experimental and initial study was approved by the Animal Ethics Committee of the University of Veterinary Medicine, Barcelona, Spain, following the specific laws of animal protection for experimentation and other specific regulations.
This new technology consisted of two roller pumps and a warm external reservoir for heating the perfusate solution, two inflow tubes, two outflow tubes and another tube used for CO2 infusion. A gas exchanger was employed for CO2 recirculation. The main advantages of this new device in the experimental study were the provision of homogeneous intra-abdominal temperature and good drug distribution (indicated by methylene blue staining) in the closed abdomen and CO2 recirculation animal group, compared with the open and closed abdomen (no CO2 recirculation) animal group. Another advantage of this new HIPEC system could be increased drug penetration through the peritoneal surface due to increased abdominal pressure, but this has not yet been demonstrated. Closed abdomen HIPEC with CO2 recirculation had no deleterious impact on blood or haemodynamic parameters in the animal study.
Once the safety and feasibility of this new technique of HIPEC had been demonstrated we started a pilot study in patients with peritoneal carcinomatosis of ovarian cancer origin, who underwent cytoreductive surgery and HIPEC with this method. The new technique may be better than classical HIPEC methods in terms of the homogeneity of the intra-abdominal temperature and drug distribution in patients undergoing cytoreductive surgery and HIPEC, with no haemodynamic perturbation. Here we present a pilot study of patients with peritoneal carcinomatosis of ovarian cancer origin who underwent complete cytoreductive surgery and HIPEC by a closed abdomen technique and CO2 recirculation using the recently developed PRS-1.0 Combat® model.
This study was approved by the Ethics Committee of the University General Hospital of Ciudad Real. The clinical trial was approved by the Spanish Medicines Agency and Medical Devices.
Objectives
The aim was to report on a series of patients with peritoneal carcinomatosis from primary or recurrent ovarian cancer who underwent cytoreductive surgery with a new type of closed HIPEC and CO2 recirculation system, using paclitaxel (175 mg/m2) for 60 min at 42 °C. We analysed the intraoperative safety as well as the post-operative safety, morbidity and mortality during the first 30 days.
Material and methods
Study population
We present the initial results of the first 21 patients included in the clinical trial EUDRACT (2011-006319-69). The main inclusion criteria for patients in this clinical trial were primary or recurrent ovarian cancer, International Federation of Gynecology and Obstetrics (FIGO) stage II, III or IV, with optimal cytoreductive surgery and no extra-abdominal metastasis. We present the first 21 patients in the HIPEC arm of the clinical trial. We performed complete cytoreductive surgery and intra-abdominal hyperthermic chemotherapy using paclitaxel (175 mg/m2) for 60 min at 42 °C. The closed abdominal HIPEC technique was used with fluid and CO2 recirculation by means of a PRS-1.0 Combat® system. Inclusion criteria were women aged 18–80 years with recurrent or primary epithelial ovarian cancer (stage II, III, or IV), performance status or functional status (according to the scale of the Eastern Cooperative Oncology Group) ≤ 2, CC0 cytoreduction rate CC1 (less than 0.25 cm), absence of extra-abdominal tumour disease; absence of cardiopulmonary, renal, or hepatic failure, provision for informed consent to participate in the study. Patients older than 80 years with a peritoneal carcinomatosis index (PCI) <10 and good performance status were assessed individually for inclusion in the study.
Perioperative management and anaesthesia
All patients received balanced general anaesthesia. In some cases an epidural catheter was used between the T7 and T8 levels for intraoperative and post-operative pain control. Invasive monitoring of blood pressure, cardiac output, and heart rate was performed by radial artery catheter pulse contour cardiac output (Pulsion® Medical Systems, Munich, Germany). A peripheral vein, a central vein and a nasogastric tube were used. Thromboprophylaxis was performed 12 h before surgery and antibiotic prophylaxis 30 min before anaesthesia induction.
Surgical technique
A midline laparotomy was performed. We performed a hysterectomy, bilateral oophorectomy, peritonectomy, and pelvic-aortic lymphadenectomy to complete macroscopically visible peritoneal implant excision. Optimal cytoreductive surgery was performed. In some patients, intestinal resection, splenectomy, or hepatic metastasis resection was necessary to achieve complete cytoreduction (). Digestive anastomosis was performed after HIPEC.
Table 1. Surgical procedures.
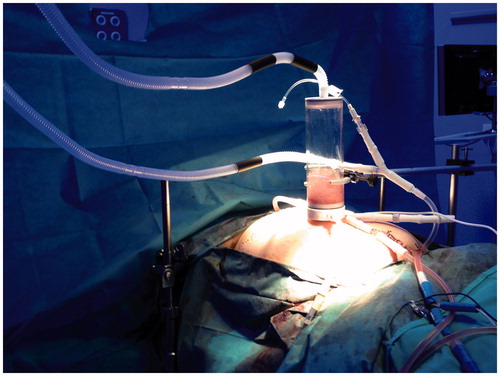
The closed abdomen HIPEC technique was performed using the PRS-1.0 Combat® system. The efficiency of the distribution of fluids and the stability and homogeneity of the intra-abdominal temperature was demonstrated previously by our group in an experimental study using a pig model [Citation5].Following cytoreductive surgery, inflow and outflow catheters and the gas exchanger were placed inside the abdominal cavity; we closed only the abdominal skin for the HIPEC procedure. The HIPEC device possessed four multi-perforated catheters, two placed in the upper abdomen (chemotherapy infusion) and two in the lower abdomen (fluid aspiration and CO2 infusion), with two roller pumps and a gas exchanger at the top of abdominal cavity. Chemotherapy with the perfusion solution at 42 °C and CO2 flowed into the abdominal cavity; turbulent flow was created to improve the drug distribution. The peritoneal dialysis solution contained 1.36% glucose and 25 mmol/L bicarbonate and 175 mg/m2 paclitaxel. HIPEC was performed for 60 min. In the animal study by our group, the CO2 infusion created turbulent flow inside the abdominal cavity, which separated all visceral faces and provided homogeneous drug distribution. In the pilot study we could not measure temperature by probe, and we only performed the same HIPEC procedure. In all cases, turbulent flow was created and there were no technological disruptions. After treatment the skin was opened and the abdominal cavity was washed and closed. All patients stayed in the intensive care unit (ICU) for the first 2–3 days.
The Sugarbaker score, i.e. the PCI, was used to define peritoneal carcinomatosis on the CT scan and during the surgical procedure. The completeness of cytoreduction (CCR-0) was defined as no macroscopic tumours. NCI-CTCAE version 4.0 was used to define the morbidity classification. The intra-abdominal temperature was evaluated using inflow and outflow temperature probes, as well as using a thermographic camera (FLIR E4,0BX, FLIR System, Kent, UK). Images were obtained during HIPEC; homogeneous colours in the thermographic images indicate a homogeneous intra-abdominal temperature. Blood samples, coagulation and arterial blood gases were taken at three time points: baseline, before HIPEC, and at the end of the procedure. The haemodynamic data were analysed at three time points during the HIPEC procedure: pre-HIPEC intra-HIPEC (at 30 min), and immediately post-HIPEC.
Statistical analysis was performed using SPSS for Windows software (version 19.0, IBM, Armonk, NY). Repeated measures analysis of variance was used to compare the means of quantitative variables taken over time from the same patient. The assumptions of normality, homoscedasticity and independence were checked, and the Friedman test was used for non-parametric data. For qualitative variables, the chi-square test was used. Differences were considered statistically significant when p < 0.05.
Results
Between November 2011 and March 2014, 21 patients with advanced epithelial ovarian cancer were included in the study. The mean age was 55.57 years (range 40–84 years). Hypertension was the most common co-morbidity (52.4% of cases, 11 patients), followed by diabetes (19% of cases, four patients). Six patients (28.6%) had previous abdominal surgery. Of the 21 patients included in the study, 16 cases (76.19%) were diagnosed with primary epithelial ovarian cancer, and five cases (23.8%) were diagnosed with recurrent ovarian epithelial cancer. FIGO stage III was the most common stage in the series (12 patients, 57.14%). Neoadjuvant chemotherapy was administered in five patients (23.8%) with carboplatin and Taxol in all cases. The PCI established by preoperative PET-CT showed considerable variability. The highest PCI was 17 (range 0–39). In 38.8% of cases the PCI by CT was greater than 10, and in 61.9% of patients the PCI by CT was less than 10. Patients with a PCI by CT lower than 10 had a higher PCI during surgery (PCI > 10). The mean time of surgery was 7 h (444.52 ± 70.74 min). The mean ICU stay was 3 days (± 1.6) and the mean hospital stay was 13.55 days (± 9.5). The analytical data collected during the procedure are shown in . There were statistically significant differences in all of the parameters studied (except for pCO2) between baseline laboratory values (before surgery, basal) and the values before HIPEC (pre-HIPEC). Statistically significant differences in bicarbonate (HCO3) and lactic acid values were found; this difference was observed between pre-HIPEC and post-HIPEC. Haemoglobin, haematocrit, prothrombin activity, pH, and pCO2 did not change after HIPEC administration. Haemodynamic parameters showed a slight increase in cardiac output and decreased peripheral vascular resistance in relation to the hyperdynamic controlled situation ().
Table 2. Blood data collected during the procedure.
Table 3. Haemodynamic data collected during the procedure.
Grade III/IV complications occurred in 38.1% and grade I/II complications occurred in 57.14% of patients (). The most frequent complications were infectious (27.27%, four cases of wound infection, a case of intra-abdominal collection treated with percutaneous drainage and one case of pneumonia) and haematological complications (22.7%) (anaemia and neutropenia). Respiratory complications occurred in 18.18% of cases; two patients had pleural effusion (thoracic drainage was necessary in one case), and one patient had an upper respiratory infection. Urological complications were observed in 18.18% of cases (pre-renal failure and urinary tract infection). Finally, 13.6% of patients had some gastrointestinal complications (post-operative ileus, nausea, or vomiting). Post-operative mortality was 4.76% (one case) due to acute respiratory distress caused by a pulmonary embolism on post-operative day 9.
Table 4. Serie morbidity.
Discussion
Intraperitoneal (IP) chemotherapy given after complete cytoreductive surgery provides increased overall survival and disease-free survival in patients with advanced or recurrent primary ovarian cancer (FIGO stages III/IV), compared with systemic chemotherapy [Citation1,Citation6,Citation7). There are many different IP treatment regimens for ovarian cancer, not only in the selection of patients, but also in cytotoxic agent (cisplatin, paclitaxel, etoposide, or carboplatin). Several studies have investigated paclitaxel-IP under normothermia. In 2013, Landrum et al. [Citation8] published the main results of prognosis factors regarding overall survival (OS) and progression-free survival (PFS) compared IP chemotherapy vs. IV chemotherapy for ovarian cancer. The median OS for IP chemotherapy patients was 61.8 months vs. 50.9 months for IV chemotherapy patients (p = 0.046). The median PFS for IP chemotherapy patients was 24.9 months vs. 20.2 months for IV chemotherapy patients (p = 0.018) [Citation8].
The most significant difference was shown by the GOG-172 (Gynecologic Oncology Group), published in 2006 by Armstrong et al. [Citation9], who compared long-term survival in peritoneal carcinomatosis from epithelial ovarian cancer (EOC) inpatients treated with intravenous (IV) therapy (paclitaxel 175 mg/m2 and cisplatin 35 mg/m2) and IV + IP therapy (paclitaxel 135 mg/m2 IV + paclitaxel 60 mg/m2 IP + cisplatin 100 mg/m2 IP). A significant survival improvement was found in the IV + IP patients compared to IV patients (overall survival of 65.6 months vs. 49.7 months, OR 0.75, p = 0.008). In the IP group, treatment was completed in only 42%, due to intra-abdominal catheter complications and chemotherapy toxicity (high dose cisplatin).
The benefit of hyperthermia in intraperitoneal chemotherapy is due to a cytotoxic effect in tumour cells. Intra-abdominal temperatures between 41–43 °C can induce apoptosis and direct DNA damage in tumour cells. Hyperthermia also enhances the cytotoxic effect of the chemotherapy agent. In ovarian cancer, paclitaxel has been used in intraperitoneal and intravenous chemotherapy by many groups, and this is the ideal cytotoxic agent in ovarian cancer, along with platinum agents. The taxane group (paclitaxel and docetaxel) have the highest molecular weight of all of chemotherapy agents (853.9 and 861.9 Daltons, respectively), and a good area under the curve ratio, so they are one of the best agents to be used in intraperitoneal chemotherapy. Paclitaxel has been used under hyperthermic conditions by some groups [Citation10–Citation14). In those studies, the authors have shown the efficacy and safety of intraperitoneal hyperthermia with paclitaxel. There are some studies that have used paclitaxel in normothermia, but there are no studies comparing both (paclitaxel hyperthermia vs. normothermia). We think that both the hyperthermia effect and the intraperitoneal paclitaxel, could improve the efficacy of oncological treatment.
The porcine model study by our group [Citation5] showed significant heat loss with the open abdomen technique. This situation could decrease the oncological efficacy of hyperthermia. A closed abdomen technique using the PRS-1.0 Combat® fluid and CO2 recirculation system showed intra-abdominal thermal homogeneity and an optimal solution distribution. Turbulent flow generates an internal agitation that achieves a homogeneous drug distribution between the visceral surfaces and recesses of the abdominal cavity, due to both the catheter distribution (on the hepatic surfaces, from right to left diaphragm) and turbulent flow. In our pig model study we demonstrated heterogeneous fluid distribution into the abdominal cavity without manual agitation. The most important point in the HIPEC technique to achieve homogeneous thermal and fluid distribution is a closed cavity, a closed circuit, and optimal agitation. With our model, we did not need to manipulate the fluid, and we obtained internal agitation and an optimal fluid distribution with no staff risk and with homogeneous intra-abdominal temperatures. In a study published in the Annals of Surgical Oncology in 2010 [Citation15], a semi-closed HIPEC technique was described in four pigs. The author obtained homogeneous intra-abdominal temperatures due to manual fluid agitation during the HIPEC process. In comparison with our experimental study, we did not need this manual agitation in the closed abdominal HIPEC group, because the turbulent flow improved solution distribution throughout the abdominal cavity. Also, in this study, despite thermal intestinal injury due to the technology used, no morbidity was observed.
This novel closed HIPEC technique, as developed in the pilot phase, could overcome the two current principal HIPEC management techniques (open or Coliseum, and closed), achieving thermal uniformity and even distribution of the drug, with little or no emission of toxic vapours.
Haemodynamic analysis
Haemodynamic alterations in patients undergoing cytoreductive surgery and HIPEC are characterised by a hyperdynamic state, determined by an increase in heart rate and cardiac output [Citation16]. Increased body temperature or possible alterations in intra-abdominal pressure during closed HIPEC might also cause haemodynamic changes [Citation17]. The PICCO® [Citation18] system used in our study offers haemodynamic monitoring by a peripheral arterial catheter and a central venous catheter. The advantages of this technology include the total volume and interstitial water measurements. Our results are similar to those of other series (the hyperdynamic state and cardiac output increases, while peripheral resistance decrease at the end of HIPEC) ().
Control of intra-abdominal pressure
With the closed HIPEC technique, some groups perform a visual abdominal filling control or use palpation of the abdominal wall to assess abdominal distension [Citation19–21]. A novel and original device has been developed by our group to control abdominal filling during HIPEC. This gas exchanger allows us to direct pressure to control intra-abdominal filling. Additionally, the intra-abdominal turbulent flow created by the solution and CO2 infusion can be visualised using this transparent device. This represents a safe and new procedure because it allows us to visualise any increase in intra-abdominal pressure (fluid level would rise on the Gas exchanger). Indirect methods to control intra-abdominal pressure include PICCO analysis and assessment of the cardiac volume at the end of diastole (GEDI) or cardiac preload values [Citation22]. No statistically significant differences in cardiac preload between the three time points analysed were observed in our series (pre-HIPEC, intra-HIPEC, and post-HIPEC, ). The increase in intra-abdominal pressure due to closed CO2 HIPEC did not affect the haemodynamic function of the patient, and we could control any haemodynamic alterations if they happened to arise [Citation23].
One factor that can improve drug penetration into the tumour is intra-abdominal pressure. An experimental animal study was performed by Gesson-Paute et al. [Citation24] comparing two groups of animals (adult pigs). In the first group HIPEC was administered by hand-assisted laparoscopic surgery, while in the other group open abdomen HIPEC was performed. Oxaliplatin absorption was higher in the laparoscopy group compared to the open surgery group. The authors concluded that the higher intra-abdominal pressure during laparoscopy increased drug penetration into the peritoneum. Also, there is a novel HIPEC treatment, pressurized intraperitoneal aerosol chemotherapy (PIPAC), which has been used in humans recently. The PIPAC method applies aerosol chemotherapy into the abdomen for 30 min at a pressure of 12 mmHg and a temperature of 37 °C [Citation25]. We used a CO2 pressure of 12 mmHg as well, but at 42 °C for 60 min. We applied a controlled high pressure, similar to the pressures used during conventional laparoscopic abdominal surgery. Both methods may improve tissue chemotherapy due to the high pressure, but in a closed HIPEC and CO2 recirculation system homogenous and optimal hyperthermia is applied, adding to the oncological thermal effect. Although we have not analysed the depth of penetration of the drug into the peritoneum, this could be a line of future research that could provide important data to this model.
The use of CO2 can be beneficial and add an oncological effect. An experimental study by Zhou et al. [Citation26] demonstrated that adding a CO2 pneumoperitoneum at 15 mmHg and 42–44° for 2–4 h induced a cytotoxic effect on gastric cancer cells induced by Bax protein, causing apoptosis. Ma et al. [Citation27] also observed a decrease in adhesion molecule expression (E-cadherin, ICAM 1, CD44 and E-selectin) in colon cancer cells after an infusion with CO2 at a pressure of 15 mmHg, both in vitro and in vivo.
Analysis of analytical variables
Anaemia and coagulopathy are the most significant haematological abnormalities during HIPEC [Citation28–30] (). We found significant differences between the basal and post-cytoreductive surgery values; however, no significant differences were found after the HIPEC procedure. Moderate metabolic acidosis and high lactic acid levels are two of the most frequent blood alterations after HIPEC. Heat-induced fluid losses, intravenous electrolyte infusion, and hyperdynamic hyperthermia may produce a hypermetabolic state, with increased oxygen consumption and changes in tissue perfusion [Citation28,Citation31]. We observed stable arterial blood pCO2 values. This new closed HIPEC model can be applied to standard laparoscopic surgeries [Citation32], but with a lower CO2 volume infusion during HIPEC. Therefore, closed HIPEC with CO2 and management technique is a reliable and safe method in gasometric terms, compared with other HIPEC techniques.
Morbidity and mortality
Major complications in our study (grade III/IV) were found in eight patients (38.1%), and minor complications (grade I/II) in 12 patients (57.14%). These results are similar to those of other studies (). Two reviews on morbidity and mortality in the treatment of peritoneal carcinomatosis of ovarian cancer origin show data from 19 and 14 studies, respectively [Citation33,Citation34]. The morbidity rates reported in these reviews were 0% and 40%, respectively, and the mean mortality rate of these studies was 3% (range 0 to 10%) [Citation37]. The results of our study show inpatients where bowel resection was performed (right or left hemicolectomy), a higher frequency of paralytic ileus (p = 0.041) and wound infection (p = 0.042). Unlike other studies, there were no cases of intestinal fistula or anastomosis dehiscence in our series. We identified no other variables that might be related to a higher rate of post-operative complications.
Figure 1. Schema device of PRS-1.0 Combat® system for closed abdomen HIPEC showing the flow direction during the procedure. A and B, roller pumps; D, gas exchanger to CO2 recirculation and visual control of filling abdominal cavity; blue keg, abdominal cavity; green line, solution and drug inflow and outflow tubes; yellow line, CO2 recirculation tube; black line, CO2 inflow tube.
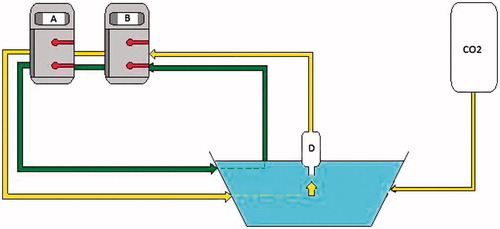
Table 5. Frequency of perioperative morbidity.
One limitation of the study was the absence of a control group in the clinical phase, where haemodynamic and laboratory data are compared between the open and closed HIPEC systems, as was conducted at the experimental stage in animals [Citation5]. Although the two phases (experimental porcine model and clinical patients) are not methodologically comparable, the animal model provided considerable information on the fluid distribution in a closed recirculating CO2-filled cavity. Despite this, the comparison between open and closed techniques in patients could represent a new line of research to corroborate our findings.
In summary, HIPEC by a closed abdomen technique with recirculation of CO2 for the treatment of advanced ovarian cancer (primary or recurrent) does not increase the number of post-operative complications compared with other studies. Together with the results of the experimental stage, we propose that this novel approach is the most appropriate technique for maintaining thermal uniformity in the peritoneal cavity and improving the distribution of the drug through the peritoneal surfaces. The oncological effectiveness of the technique was shown by an increase in overall survival or progression-free survival in these patients. This hypothesis was assessed after completion of the clinical trial EUDRACT 2011-006319-69, currently in development at our hospital.
Acknowledgements
Special acknowledgements go to the surgical staff who made this trial possible.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jaaback K, Johnson N, Lawrie TA. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 2011;11:CD005340.
- Overgaard J. Effect of hyperthermia on malignant cells in vivo. A review and a hypothesis. Cancer 1977;39:2637–46.
- El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia 2004;6:117–27.
- Glehen O, Cotte E, Kusamura S, Deraco M, Baratti D, Passot G, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol 2008;98:242–6.
- Sanchez-Garcia S, Padilla-Valverde D, Villarejo-Campos P, Martin-Fernandez J, Garcia-Rojo M, Rodriguez-Martinez M. Experimental development of an intra-abdominal chemohyperthermia model using a closed abdomen technique and a PRS-1.0 Combat CO2 recirculation system. Surgery 2014;155:719–25.
- Jaaback K, Johnson N. Intraperitoneal chemotherapy for the initial management of primary epithelial ovarian cancer. Cochrane Database Syst Rev 2006;CD005340.
- Elit L, Oliver TK, Covens A, Kwon J, Fung MF, Hirte HW, et al. Intraperitoneal chemotherapy in the first-line treatment of women with stage III epithelial ovarian cancer: a systematic review with metaanalyses. Cancer 2007;109:692–702.
- Landrum LM, Java J, Mathews CA, Lanneau GS Jr, Copeland LJ, Armstrong DK, Walker JL. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol 2013;130:12–18.
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34–43.
- de Bree E, Rosing H, Filis D, Romanos J, Melisssourgaki M, Daskalakis M, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol 2008;15:1183–92.
- Kim JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Consolidation hyperthermic intraperitoneal chemotherapy using paclitaxel in patients with epithelial ovarian cancer. J Surg Oncol 2010;101:149–55.
- Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol 2007;106:193–200.
- Cascales Campos PA, Gil Martinez J, Galindo Fernandez PJ, Gil Gomez E, Martinez Frutos IM, Parrilla Paricio P. Perioperative fast track program in intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) after cytoreductive surgery in advanced ovarian cancer. Eur J Surg Oncol 2011;37:543–8.
- Rufian S, Munoz-Casares FC, Briceno J, Diaz CJ, Rubio MJ, Ortega R, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol 2006;94:316–24.
- Ortega-Deballon P, Facy O, Magnin G, Piard F, Chauffert B, Rat P. Using a heating cable within the abdomen to make hyperthermic intraperitoneal chemotherapy easier: feasibility and safety study in a pig model. Eur J Surg Oncol 2010;36:324–8.
- Esquivel J, Angulo F, Bland RK, Stephens AD, Sugarbaker PH. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open ‘Coliseum technique’. Ann Surg Oncol 2000;7:296–300
- Kanakoudis F, Petrou A, Michaloudis D, Chortaria G, Konstantinidou A. Anaesthesia for intra-peritoneal perfusion of hyperthermic chemotherapy. Haemodynamic changes, oxygen consumption and delivery. Anaesthesia 1996;51:1033–6.
- Litton E, Morgan M. The PiCCO monitor: a review. Anaesth Intensive Care 2012;40:393–409.
- Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, et al. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg 2007;31:1813–20.
- Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315–25.
- Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754–63.
- Cheatham ML. Abdominal compartment syndrome: pathophysiology and definitions. Scand J Trauma Resusc Emerg Med 2009;17:10.
- Facy O, Combier C, Poussier M, Magnin G, Ladoire S, Ghiringhelli F, Chaufert B, et al. High pressure does not counterbalance the advantages of open techniques over closed techniques during heated intraperitoneal chemotherapy with oxaliplatin. Surgery 2015;157:72–8.
- Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D. Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol 2008;15:339–44.
- Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, et al. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 2014;21:553–9.
- Zhou HM, Feng B, Zhao HC, Zheng MH. Antitumor effects of hyperthermic CO2 pneumoperitoneum on human gastric cancer cells. Asian Pac J Cancer Prev 2012;13:117–22
- Ma JJ, Feng B, Zhang Y, Li JW, Lu AG, Wang ML, et al. Higher CO2-insufflation pressure inhibits the expression of adhesion molecules and the invasion potential of colon cancer cells. World J Gastroenterol 2009;15:2714–22.
- Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008;63:389–95.
- Cooksley TJ, Haji-Michael P. Post-operative critical care management of patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC). World J Surg Oncol 2011;9:169.
- Bell JC, Rylah BG, Chambers RW, Peet H, Mohamed F, Moran BJ. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol 2012;19:4244–51.
- Webb CA, Weyker PD, Moitra VK, Raker RK. An overview of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for the anesthesiologist. Anesth Analg 2013;116:924–31.
- Demiroluk S, Salihoglu Z, Bakan M, Bozkurt P. Effects of intraperitoneal and extraperitoneal carbon dioxide insufflation on blood gases during the perioperative period. J Laparoendosc Adv Surg Tech A 2004;14:219–22.
- Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol 2009;135:1637–45.
- Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol 2007;18:1943–50.
- Pavlov MJ, Kovacevic PA, Ceranic MS, Stamenkovic AB, Ivanovic AM, Kecmanovic DM. Cytoreductive surgery and modified heated intraoperative intraperitoneal chemotherapy (HIPEC) for advanced and recurrent ovarian cancer – 12-year single center experience. Eur J Surg Oncol 2009;35:1186–91.
- Fagotti A, Paris I, Grimolizzi F, Fanfani F, Vizzielli G, Naldini A, et al. Secondary cytoreduction plus oxaliplatin-based HIPEC in platinum-sensitive recurrent ovarian cancer patients: a pilot study. Gynecol Oncol 2009;113:335–40.
- Helm CW, Bristow RE, Kusamura S, Baratti D, Deraco M. Hyperthermic intraperitoneal chemotherapy with and without cytoreductive surgery for epithelial ovarian cancer. J Surg Oncol 2008 15;98:283–90.