Abstract
Purpose: Oesophageal cancer is a highly aggressive disease with about 50% of patients presenting with advanced or metastatic disease at initial diagnosis. In this study we assessed combined microwave ablation (MWA) and systemic chemotherapy in the treatment of liver metastases arising from oesophageal squamous cell carcinoma (OSCC). Materials and methods: Between February 2009 and June 2014, OSCC patients who underwent percutaneous MWA + concurrent systemic chemotherapy and systemic chemotherapy alone for liver metastases were enrolled in this study. Overall survival (OS) and progression-free survival (PFS) were recorded and compared between groups. Results: In total 15 patients with 25 liver metastases who underwent ultrasound-guided percutaneous MWA and chemotherapy were enrolled in this study. Technical success was achieved in 96% (24/25) of metastatic liver tumours. No major or minor complications associated with MWA procedures were observed. The median OS and PFS from initial MWA were 13 months and 4 months. The 1-, 2-, 3-, 4-year OS rates after MWA were 53.3%, 26.7%, 13.3%, and 13.3%, respectively. The 1- and 2-year PFS rates after MWA were 26.7% and 13.3%. The OS and PFS of the MWA + systemic chemotherapy group were superior than those of patients who received systemic chemotherapy alone (P = 0.011 and 0.030, respectively). Conclusions: Combined MWA with systemic chemotherapy is a feasible, safe and effective treatment for liver metastases from OSCC.
Introduction
Oesophageal cancer led to about 400,000 deaths worldwide in 2012, ranking as the sixth leading cause of cancer deaths [Citation1]. About 90% of worldwide oesophageal cancer cases are oesophageal squamous cell carcinoma (OSCC) [Citation2]. Almost 50% oesophageal cancer patients present with advanced or metastatic disease at initial diagnosis, mainly due to its aggressive biology [Citation3], and the 5-year survival of patients with advanced or metastatic oesophageal cancer is just 3–5% [Citation4]. The most common distant-organ metastases of oesophageal cancer are lung, liver and bone [Citation5]. Recent studies have indicated that patients with liver recurrence after oesophagectomy had worse survival than those with other distant-organ metastases [Citation5,Citation6]. Consequently, local control of liver metastases from oesophageal cancer (OCLM) has significant implications.
In recent years, multimodality treatments have been investigated for liver metastases, including surgical and non-surgical approaches [Citation7]. In oesophageal cancer, as most patients with liver metastases are not surgical candidates, palliative systemic chemotherapy and radiotherapy is the mainstay for the treatment of OCLM. Cisplatin + fluorouracil (CF) regimen is the most commonly used salvage treatment for metastatic oesophageal cancer [Citation8], with a reported response rate of 13–35.9% [Citation9]. Considering the low resection rate and the unsatisfactory outcome of systemic chemotherapy, effective loco-regional treatment is thus urgently needed to obtain local control and improve survival for patients with OCLM.
Nowadays, ablative therapies have been considered a promising treatment for metastatic liver malignancies due to minimal invasiveness, effectiveness and low rate of complications [Citation10]. Our previous studies have demonstrated the safety and effectiveness of microwave ablation (MWA) in the treatment of colorectal cancer liver metastases [Citation11]. In this study we investigated the feasibility, safety and efficacy of combined MWA and systemic chemotherapy for OCLM. To the best of our knowledge, no results have been reported regarding the feasibility and safety of MWA for unresectable OCLM.
Materials and methods
This study received approval from the Chinese PLA General Hospital Institutional Review Board. Each patient signed informed consent for treatment procedures before therapy.
Patient selection
We prospectively analysed the database of patients who underwent MWA and concurrent systemic chemotherapy for OCLM at our department between February 2009 and June 2014. The database included the medical records, radiological imaging, and laboratory tests of each patient before, during MWA treatment and during the follow-up. The decision of MWA treatment for OCLM was made after a case-by-case evaluation based on patients’ clinico-pathological characteristics and radiological results. The inclusion criteria of this study were as follows: patients who had undergone oesophagectomy for OSCC; radiological imaging- or biopsy-confirmed metachronous liver metastases; patients either ineligible for liver resection or refusing surgery; Karnofsky Performance Scale (KPS) score of 70 or more; three or fewer liver metastases with a maximum diameter of 5 cm; no severe coagulopathy (e.g. platelet count <50 × 109/L, or prolonged prothrombin time >30 s); MWA could be performed with a curative intent. Patients with evidence of portal vein cancerous thrombosis, extrahepatic metastases, and patients who received liver-directed therapies such as transcatheter arterial chemoembolisation before ablation were excluded.
Meanwhile, we reviewed the database of patients who received only systemic chemotherapy for OCLM after resection of OSCC during the same period. Patients in systemic chemotherapy group were not candidates for surgical resection and had no evidence of portal vein cancerous thrombosis or extrahepatic metastases. The treatment regimen was determined by the consensus of the multidisciplinary tumour panel, which consisted of experienced surgeons, oncologists, and radiologists, following clinical assessment and radiological findings. The patients received systemic chemotherapy based on the location and extent of the primary cancer, as well as the patients’ physical condition. The age, sex, KPS score, location, histological grade, TNM stage of primary oesophageal cancer, interval from oesophagectomy to liver metastases, carcinoembryonic antigen level before treatment, location, number, size of liver metastases, and type of systemic chemotherapy for each patient were recorded.
MWA procedures
Two experienced interventional radiologists (X.L.Y., 22 years of experience in MWA and P.L., 22 years of experience) performed the MWA. Indications, risks and advantages of MWA were discussed with each patient before treatment. Before therapy, contrast-enhanced ultrasound (CEUS, SonoVue, Bracco, Milan, Italy) was performed in each patient to better display the number, size and location of OCLM. A cooled-shaft microwave system (KY-2000, Kangyou Medical, Nanjing, China) with a frequency of 2450 MHz and a maximum power of 100 W was administered. After intravenous anaesthesia with propofol (6–12 mg/kg/h) and ketamine (1–2 mg/kg) by an anaesthesiologist, microwave antennae were inserted under conventional ultrasound or CEUS guidance. One antenna was inserted at the designated location for tumours with diameters less than 1.7 cm, whereas two antennae were used for tumours with diameters of 1.7 cm or more. Microwaves were emitted with a routine output setting of 50–60 W for 5–10 min during one session based on our previous study on thermal field [Citation12]. To monitor real-time temperature, a 20-gauge thermocouple was placed at the tumour margin under ultrasound guidance. MWA treatment was stopped when the hyperechoic area covered the entire tumour with a safety-margin of at least 5 mm, or the measured temperature remained above 54 °C for more than 3 min, or reached 60 °C. After ablation the antennae were gradually withdrawn, and microwave emission was continued to prevent bleeding and tumour seeding. If remaining contrast enhancement was displayed in the rim or in the context of ablated tumour on CEUS immediately after ablation, additional MWA sessions were required until complete ablation. Technical success was achieved if ‘complete ablation’ was displayed on CEUS 1 month after MWA. Complications were categorised as major or minor according to the criteria proposed by the Society of Interventional Radiology Technology Assessment Committee and the International Working Group on Image-guided Tumor Ablation [Citation13]. A validated visual analogue scale (VAS) was applied to evaluate the pain grade.
Follow-up
CEUS and contrast-enhanced magnetic resonance imaging (MRI) were performed 1, 3, 6, 9 and 12 months after initial MWA/systemic chemotherapy and at 3–6-month intervals thereafter. One patient was administered CEUS and contrast-enhanced computed tomography (CT) during the follow-up due to the implantation of a heart stent. Additionally, laboratory tests were performed at the same time. Local recurrence was defined as the presence of viable tumour inside or adjacent to the ablated tumour at CEUS or other imaging modalities at least 1 month after MWA. Follow-up period was recorded as the time from initial MWA to the most recent CEUS scan.
Statistical analysis
Overall survival (OS) was defined as the time from initial MWA/systemic chemotherapy to death. Progression-free survival (PFS) was defined as the time from initial MWA/systemic chemotherapy to any sign of disease progression (intrahepatic or extrahepatic recurrence) or death. OS and PFS curves were described with the Kaplan–Meier method. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA).
Results
Demographics
From 2009–2014, a total of 122 patients received MWA for OCLM at our department. After exclusion of patients who failed to meet inclusion criteria, ultimately 15 patients with 25 liver metastases who underwent ultrasound-guided percutaneous MWA and systemic chemotherapy were enrolled in this study (). The clinico-pathological characteristics of enrolled patients are summarised in . Two women and 13 men aged 44–78 years (mean ± SD 59 ± 9 years) at initial MWA were enrolled. The location of primary OSCC were upper thoracic oesophagus (n = 1), middle thoracic oesophagus (n = 3), and lower thoracic oesophagus (n = 11). The median carcinoembryonic antigen level before MWA was 2.60 μg/L (range 0.84–138.5 μg/L). Thirteen patients were confirmed through ultrasound-guided biopsy, while the remaining two refused biopsy and were confirmed through CEUS and contrast-enhanced MRI. Median interval from oesophagectomy to detection of liver metastases was 10 months (range 3–53 months). All the patients received concurrent systemic chemotherapy with MWA based on the location, stage of OSCC and the patients’ performance status. Systemic chemotherapy administered in this group included CF regimen (n = 9) and docetaxel + cisplatin + fluorouracil (DCF) regimen (n = 6).
Figure 1. Enrolment of patients who received microwave ablation for liver metastases from oesophageal squamous cell carcinoma at our department.
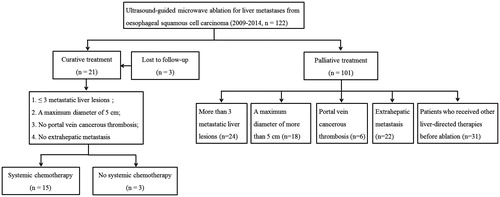
Table 1. Clinicopathological characteristics of 15 patients with liver metastases from oesophageal squamous cell carcinoma.
In the systemic chemotherapy group, 26 patients who met inclusion criteria were enrolled – 19 men and 7 women. Multiple intrahepatic metastatic lesions were detected in 16 patients and the mean largest diameter of liver metastases was 4.8 ± 2.1 cm (range 2.6–9.8 cm). Systemic chemotherapy regimens included CF regimen (n = 15), DCF regimen (n = 8), and epirubicin + cisplatin + fluorouracil (ECF) regimen (n = 3).
Liver metastases and MWA characteristics
Parameters for liver metastases and MWA treatment are listed in . The number of liver metastases was one in seven patients, two in six patients and three in two patients. Mean ablation power and times were 51.2 ± 3.3 W (range 50–60 W) and 439.6 ± 213.1 s (range 150–780 s). Fourteen patients underwent MWA procedures under conventional ultrasound guidance, and one patient was under CEUS guidance with tumours inconspicuous on conventional ultrasound. Three patients were treated with one microwave antenna, and 12 patients were treated with two antennae. Technical success (complete ablation) was achieved in 24/25 (96%) tumours, and the remaining tumour underwent additional MWA sessions to obtain complete ablation. No major or minor complications associated with the MWA procedure were observed. Side effects observed included self-limiting fever (n = 3), malaise (n = 4), and mild pain of the right upper quadrant (n = 3).
Table 2. Parameters of liver metastases and microwave ablation treatment
Follow-up and survival
The patients in the MWA group were followed up for a median of 14 months (range 2–48 months) since initial MWA. The median OS and PFS times from initial MWA were 13 months (95% confidence interval (95% CI) 9.2–16.8 months) and 4 months (95% CI 1.0–7.0 months). The 1-, 2-, 3-, and 4-year OS rates after MWA were 53.3%, 26.7%, 13.3%, and 13.3%, respectively (). The 1- and 2-year PFS rates after MWA were 26.7% and 13.3% (). Patients developed local recurrence (n = 2), intrahepatic recurrence of remnant liver (n = 6), and extrahepatic metastases of lymph node (n = 6), lung (n = 4), brain (n = 2), kidney (n = 1), and adrenal gland (n = 1) after ablation. Of note, five patients received repeat MWA for recurrent intrahepatic metastases during the follow-up and two patients (no. 1 and no. 3) were still alive 19 and 48 months after initial MWA, respectively. Patient no. 15 remained progression-free 24 months after initial MWA. Until the last follow-up, three patients were alive and 12 had died due to disease progression. The mean interval from oesophagectomy to liver metastases, mean number and size of liver metastases of patients who were alive/dead were 33/14.7 months, 1.3/1.8 and 2.95/3.08 cm. The clinical characteristics of the three patients who were alive were listed in .
Figure 2. Overall survival and progression-free survival of 15 patients after combined microwave ablation and systemic chemotherapy for liver metastases from oesophageal squamous cell carcinoma.
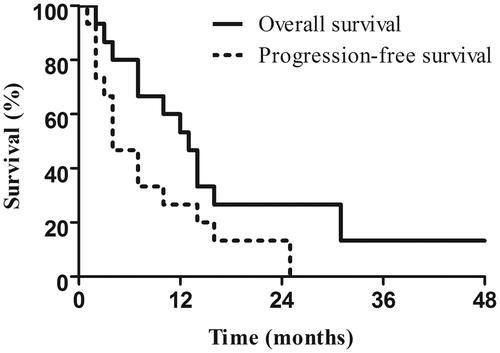
Table 3. Clinical characteristics of three patients who were still alive at the last follow-up.
In the systemic chemotherapy group, the median OS was 7 months (95% CI 5.0–9.0 months) with a 1-year OS rate of 11.5%. The median PFS was 4 months (95% CI 3.1–4.9 months). OS result of the systemic chemotherapy group was inferior in comparison with that of the MWA group (hazard ratio (HR) 0.381, 95% CI 0.180–0.805; P = 0.011). Similarly, the systemic chemotherapy group showed worse PFS outcome than that of the MWA group (HR 0.422, 95% CI 0.194–0.920; P = 0.030).
Discussion
In this study technical success of MWA was achieved in 96% of metastatic tumours. No procedure-related deaths, major or minor complications were reported. The median OS time from initial MWA was 13 months with the 1-, 2-, 3-, and 4-year OS rates of 53.3%, 26.7%, 13.3%, and 13.3%, respectively. The median PFS time from initial MWA was 4 months with 1- and 2-year PFS rates of 26.7% and 13.3%. Five patients received repeat MWA for recurrent intrahepatic metastases during follow-up. OS and PFS of MWA + systemic chemotherapy group were significantly better than those of the systemic chemotherapy group. Consequently, we suggest that a combination of MWA and systemic chemotherapy could serve as an alternative to reduce tumour burden and prolong survival in strictly selected patients with unresectable OCLM.
According to previous studies, patients with liver metastases after oesophagectomy had significantly worse post-recurrence survival (HR = 2.255, P = 0.032) [Citation6], shorter progression-free survival time (2.4 months versus 5.4 months, P = 0.003) [Citation9], and worse overall survival (5 months versus 13 months, P-value not shown) than those with other distant-organ metastases [Citation5]. Consequently, local control of OCLM has important implications.
In synchronous OCLM, palliative systemic chemotherapy (mainly CF regimen) has been regarded as a standard salvage treatment [Citation8], with a response rate of 13–35.9%, a median PFS of 3.6 months and a median survival of 5.5–6.7 months [Citation9]. Over the past decade, molecular-targeted agents that target vascular endothelial growth factor or its receptor, the cyclooxygenase-2 epidermal growth factor receptor, and mammalian target of rapamycin pathways have been explored for metastatic oesophageal cancer [Citation14,Citation15]. Until now, the number of approved targeted agents remains few and whether these agents in combination with chemotherapy regimen improve efficacy over chemotherapy alone still needs further investigation. Synchronous resection of both primary and metastatic tumour is not commonly administered as a large proportion of patients are not candidates for hepatectomy due to their poor general condition, tumour invasion, extrahepatic disease or lymph node involvement [Citation16].
In those who developed liver metastases after oesophagectomy, the optimal treatment has yet to be fully evaluated [Citation9,Citation17,Citation18]. A few studies have reported treatment for metachronous OCLM, and are shown in . In addition, a series of case reports have been published in Japanese regarding different treatment modalities for metachronous OCLM (discussed below).
Table 4. Summary of literature regarding treatment for metachronous liver metastases from oesophageal cancer.
Andreou et al. reported a 5-year OS rate of 25% after hepatectomy for OCLM (n = 4, ) [Citation17]. In our study, the 4-year OS rate after MWA was 13.3%. These differences may be attributed to different modalities (surgery versus MWA), the small number of studies, and/or different histologic type and location of the primary tumour.
Huddy et al.[Citation4] reported four patients who underwent liver resection for OCLM and deduced that the number of liver metastases, the disease-free interval before development of liver metastases, and response to prior chemotherapy may predict tumour biology and provide valuable clues on the decision for liver resection [Citation4]. In our study, patients who were still alive had a relatively longer interval from oesophagectomy to liver metastases (33 months versus 14.7 months), a smaller number of metasteses (1.3 versus 1.8), and a larger size of liver metastases (2.95 cm versus 3.08 cm) than those who had died by the last follow-up. Though the number in this study is very small, we could hypothesize that the above factors may to some extent lead to a relatively longer survival after MWA. However, whether these are true prognostic factors for survival needs further studies to clarify.
Ichida and colleagues reported a median survival of 13 months after hepatectomy for OCLM (n = 5, ) [Citation5]. In the current study, the median survival after MWA was 13 months, comparable with that of Ichida et al.’s study. Our results suggest that in selected patients, MWA combined with systemic chemotherapy may serve as an alternative to hepatectomy for unresectable OCLM, and may achieve comparable survival outcome with that of surgical resection (13 months versus 13 months) [Citation5].
Systemic chemotherapy is commonly administered for OCLM. Wang et al. investigated the efficacy of FOLFOX regimen (oxaliplatin, leucovorin and fluorouracil) for OCLM after oesophagectomy (n = 20, ) [Citation9]. Median OS and PFS were 6.7 months and 2.4 months. In Muro et al.’s study, 11 patients received systemic docetaxel for liver metastases, and the response rate was 18% () [Citation19]. In a case report of a patient with liver metastases from oesophageal adenosquamous carcinoma, systemic chemotherapy of CF regimen was administered and effective local control was achieved [Citation20]. In the systemic chemotherapy group of this study, regimens administered included CF, DCF, and ECF. OS of the systemic chemotherapy group was comparable with that of Wang and colleagues’ research (7 months versus 6.7 months), while PFS was better than theirs (4 months versus 2.4 months).
Except for surgical resection and systemic chemotherapy, other treatments have been reported to achieve local control for OCLM. These therapies include hepatic arterial infusion (HAI) of CF regimen (n = 8, overall response rate of 50%) [Citation21] (), HAI of autologous tumour cell-activated T lymphocytes (n = 2, both achieved clinical regression) [Citation22], and conformal radiotherapy (n = 1, effective local control) () [Citation18].
As oesophageal cancer is a highly aggressive disease, monotherapy may not obtain satisfactory results. Nowadays, multimodality treatment has been investigated for OCLM. Reported treatments include systemic docetaxel + radiofrequency ablation + hepatectomy () [Citation14], HAI of CF regimen + hyperthermotherapy () [Citation23], hepatic resection + systemic chemotherapy (FAP) + HAI chemotherapy (FAP) [Citation24], systemic chemotherapy (CF regimen) + transarterial chemoembolisation [Citation24], stereotactic irradiation + transarterial chemoembolisation [Citation25], stereotactic irradiation + systemic chemotherapy (docetaxel + nedaplatin) [Citation26], HAI chemotherapy (CF regimen) + radiation therapy + liver resection [Citation27], intra-arterial 5-flourouracil chemotherapy [Citation28], chemoradiotherapy (docetaxel hydrate + S-1 + radiation) [Citation29]. However, the above studies were mostly case reports, and studies of a larger population are still needed.
In recent years MWA has been developed as a promising modality due to its minimal invasiveness, effectiveness, repeatability and low rate of complications. The efficacy of MWA has been demonstrated in patients with primary and metastatic liver malignancies [Citation10,Citation11]. In our study, all the patients with unresectable OCLM received MWA combined with systemic chemotherapy and they obtained better survival outcomes than those who received systemic chemotherapy alone. This combined treatment provides the following benefits. Firstly, the combination of MWA may increase the proportion of patients with unresectable OCLM who may obtain local control. In this study, the rate of complete ablation reached 96%. In the past, patients with OCLM adjacent to risk locations (e.g. large vessels, liver hilum, gallbladder, diaphragm, gastrointestinal tract) were considered ‘unresectable’ and could only resort to systemic chemotherapy for palliative intent. Nowadays, the safety and efficacy of MWA has been confirmed in patients with liver malignancies adjacent to these risk locations [Citation30–33]. A preliminary study in our department has demonstrated the safety of MWA in patients with renal dysfunction, when these patients would have been categorised as ‘unresectable’ in the past [Citation34]. Additionally, the repeatability and low rate of complications of MWA offers a chance of longer survival for patients with recurrent liver metastases. In this study no procedure-related major or minor complications were reported and five patients received repeat MWA for recurrent liver metastases after initial ablation, which indicated the willingness of patients to receive further treatment during the follow-up. Based on our results, we suggest that MWA be included in the multimodality treatment for unresectable OCLM after evaluation by a multidisciplinary tumour panel, though the results still need further investigation in a larger population.
Our study has several limitations. Firstly, this is a retrospective study with selection bias. Therefore, the results may represent a selected population of OCLM patients in China. Secondly, the number of patients enrolled in this study is very small. Most patients had extensive disease when they came to our department and could only achieve a palliative intent after MWA, which led to the small number of patients enrolled. Thirdly, though all the patients in the two groups had unresectable liver-only metastases, patients in the two groups were not comparable. The reason may be that patients in the MWA group could achieve curative intent after ablation while those in the systemic chemotherapy could only achieve palliative intent after discussion by a multidisciplinary tumour panel. Consequently, randomised controlled trials are needed to better evaluate the efficacy of MWA for OCLM.
Conclusion
To conclude, combined percutaneous MWA with systemic chemotherapy is a feasible, safe and effective loco-regional treatment of liver metastases from OSCC. The minimal invasiveness, repeatability, and low rate of complications of MWA indicate that combination of MWA and systemic chemotherapy may serve as an alternative in selected patients with unresectable liver metastases from OSCC.
Acknowledgements
F.Z. and X.Y. contributed equally to this paper and both should be considered as first authors. The authors thank all the enrolled patients in this study.
Disclosure statement
This research was funded by the National Natural Science Foundation of China (no. 81171358, no. 81471683, no. 81127006 and no. 81430039). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86.
- Feng XS, Yang YT, Gao SG, Ru Y, Wang GP, Zhou B, et al. Prevalence and age, gender and geographical area distribution of esophageal squamous cell carcinomas in North China from 1985 to 2006. Asian Pac J Cancer Prev 2014;15:1981–7.
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499–509.
- Huddy JR, Thomas RL, Worthington TR, Karanjia ND. Liver metastases from esophageal carcinoma: is there a role for surgical resection? Dis Esophagus 2015;28: 483–7.
- Ichida H, Imamura H, Yoshimoto J, Sugo H, Kajiyama Y, Tsurumaru M, et al. Pattern of postoperative recurrence and hepatic and/or pulmonary resection for liver and/or lung metastases from esophageal carcinoma. World J Surg 2013;37:398–407.
- Hsu PK, Wang BY, Huang CS, Wu YC, Hsu WH. Prognostic factors for post-recurrence survival in esophageal squamous cell carcinoma patients with recurrence after resection. J Gastrointest Surg 2011;15:558–65.
- Choti MA. Liver-directed treatments for metastatic colorectal cancer. Curr Treat Options Oncol 2014;15:456–64.
- Hironaka S, Tsubosa Y, Mizusawa J, Hironaka S, Tsubosa Y, Mizusawa J, et al. Phase I/II trial of 2-weekly docetaxel combined with cisplatin plus fluorouracil in metastatic esophageal cancer (JCOG0807). Cancer Sci 2014;105:1189–95.
- Wang J, Chang J, Yu H, Wu X,Wang H, Li W, et al. A phase II study of oxaliplatin in combination with leucovorin and fluorouracil as first-line chemotherapy in patients with metastatic squamous cell carcinoma of esophagus. Cancer Chemother Pharmacol 2013;71:905–11.
- Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation – complications among cohort of 1136 patients. Radiology 2009;251:933–40.
- Wang J, Liang P, Yu J, Yu MA, Liu F, Cheng Z, et al. Clinical outcome of ultrasound-guided percutaneous microwave ablation on colorectal liver metastases. Oncol Lett 2014;8:323–26.
- Liang P, Dong B, Yu X, Yu D, Cheng Z, Su L, et al. Computer-aided dynamic simulation of microwave-induced thermal distribution in coagulation of liver cancer. IEEE Trans Biomed Eng 2001;48:821–9.
- Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. J Vasc Interv Radiol 2014;25:1691–705.
- Iitaka D, Shiozaki A, Fujiwara H, Ichikawa D, Okamoto K, Komatsu S, et al. Case involving long-term survival after esophageal cancer with liver and lung metastases treated by multidisciplinary therapy: report of a case. Surg Today 2013;43:556–61.
- Mohamed A, El-Rayes B, Khuri FR, Saba NF. Targeted therapies in metastatic esophageal cancer: advances over the past decade. Crit Rev Oncol Hematol 2014;91:186–96.
- Mudan SS, Giakoustidis A, Giakoustidis D, Slevin M. Synchronous oesophagectomy and hepatic resection for metastatic oesophageal cancer: report of a case. Hippokratia 2010;14:291–3.
- Andreou A, Viganò L, Zimmitti G, Seehofer D, Dreyer M, Pascher A, et al. Response to preoperative chemotherapy predicts survival in patients undergoing hepatectomy for liver metastases from gastric and esophageal cancer. J Gastrointest Surg 2014;18:1974–86.
- Ikeda Y, Niimi M, Kan S, Shatari T, Takami H, Kodaira S. Conformal radiation therapy for liver metastasis of esophageal carcinoma. Hepatogastroenterology 2003;50:532–4.
- Muro K, Hamaguchi T, Ohtsu A, Boku N, Chin K, Hyodo I, et al. A phase II study of single-agent docetaxel in patients with metastatic esophageal cancer. Ann Oncol 2004;15:955–9.
- Uchikado Y, Natsugoe S, Okumura H, Matsumoto M, Setoyama T, Takigawa J, et al. A case of successful treatment by low-dose 5-fluorouracil and cisplatin for liver metastases of esophageal adenosquamous carcinoma. Gan To Kagaku Ryoho 2006;33:83–5.
- Nakajima Y, Nagai K, Kawano T, Inoue H, Nara S, Kumagai Y, et al. Therapeutic strategy for postoperative liver metastasis from esophageal squamous cell carcinoma; clinical efficacy of and problem with hepatic arterial infusion chemotherapy. Hepatogastroenterology 2001;48:1652–5.
- Toh U, Sudo T, Kido K, Tanaka T, Sueyoshi S, Fujita H, et al. Intraarterial cellular immunotherapy for patients with inoperable liver metastases of esophageal cancer. Gan To Kagaku Ryoho 2002;29:2152–6.
- Iwahashi M, Tanimura H, Nakamori M, Nagai Y, Hirabayashi N, Ueda K, et al. Clinical evaluation of hepatic arterial infusion of low dose-CDDP and 5-FU with hyperthermotherapy: a preliminary study for liver metastases from esophageal and gastric cancer. Hepatogastroenterology 1999;46:2504–10.
- Nagano H, Sakon M, Yasuda T, Dono K, Nakamori S, Yano M, et al. A case of postoperative multiple hepatic metastasis from esophageal cancer successfully treated by surgical resection and hepatic arterial infusion chemotherapy. Gan To Kagaku Ryoho 2001;28:1628–31.
- Wada N, Yamasaki M, Hayashi T, Miyata H, Takahashi T, Kurokawa Y, et al. Clinical experience of topical treatments for liver metastases from esophageal carcinoma. Gan To Kagaku Ryoho 2013;40:2158–60.
- Egawa T, Okubo Y, Kemmochi T, Mori T, Sato S, Nishiya S, et al. A case of liver metastasis from esophageal cancer treated with stereotactic body radiation therapy. Gan To Kagaku Ryoho 2013;40:1850–2.
- Ikebe M, Kitamura M, Saitoh G, Hasegawa H. Multimodality therapy containing hepatic arterial infusion chemotherapy for liver metastasis of esophageal cancer – a case report. Gan To Kagaku Ryoho 2012;39:1555–7.
- Shuto K, Ohira G, Kono T, Natsume T, Tohma T, Sato A, et al. Regional treatment of esophageal liver metastasis by intra-arterial low-dose 5-FU therapy. Gan To Kagaku Ryoho 2010;37:2409–11.
- Kubota H, Matsumoto H, Saito A, Kaida Y, Murakami H, Higashida M, et al. A case of chemo-radiation therapy with high degree of efficacy for esophageal cancer with liver metastasis. Gan To Kagaku Ryoho 2009;36:2045–8.
- Li M, Yu X, Liang P, Dong B, Liu F. Ultrasound-guided percutaneous microwave ablation for hepatic malignancy adjacent to the gallbladder. Int J Hyperthermia 2015;31:579–87.
- Zhang D, Xie D, Wei X, Zhang D, Chen M, Yu X, et al. Microwave ablation of the liver abutting the stomach: insulating effect of a chitosan-based thermosensitive hydrogel. Int J Hyperthermia 2014;30:126–33
- Zhang D, Liang P, Yu X, Cheng Z, Han Z, Yu J, et al. The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia 2013;29:663–70.
- Li M, Yu XL, Liang P, Liu F, Dong B, Zhou P. Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 2012;28:218–26.
- Liu C, Wang Y, Yu X, Dong B, Zhou P, Ren H, et al. Is percutaneous microwave ablation of liver tumor safe for patients with renal dysfunction. Eur J Radiol 2011;79:e103–7.
