Abstract
Context: Bladder cancer therapy remains suboptimal as morbidity and mortality remain high amongst those with non-muscle-invasive and muscle-invasive disease. Regional hyperthermia therapy (RHT) is a promising adjunctive therapy being tested in multiple clinical contexts.
Objective: The aim of this study was to systematically review the literature on the efficacy and toxicity of RHT.
Evidence acquisition: This systematic review was registered with the PROSPERO database (Registration number: CRD42015025780) and was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. We queried PubMed, EMBASE, and Cochrane libraries. Two reviewers reviewed abstracts independently and a third reviewer arbitrated disagreements. The last search was performed on 28 August 2015. A descriptive analysis was performed and quality assessment was conducted using the Newcastle-Ottawa Quality Assessment Scale for observational studies, and the Cochrane Risk of Bias Assessment Tool for trials.
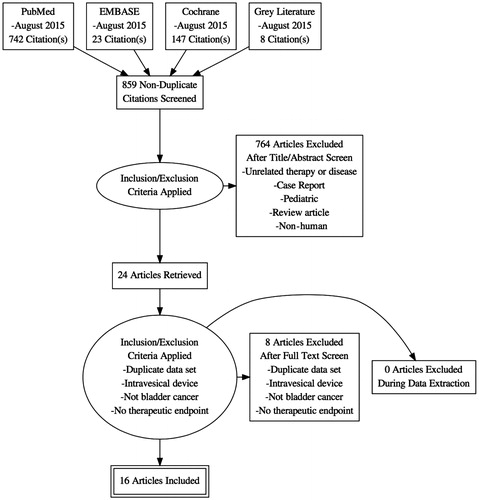
Evidence synthesis: We identified 859 publications in the initial search, of which 24 met inclusion criteria for full-text review. Of these, we were able to obtain data on the outcomes of interest for 15 publications.
Conclusions: The review underscores the limited nature of the evidence; definitive conclusions are elusive. However, the promising results of RHT in the setting of intravesical chemotherapy, chemotherapy and radiotherapy show a trend towards legitimate efficacy.
Introduction
There will be an estimated 74,000 new cases of bladder cancer and 16,000 deaths in the USA in 2015. This represents 4.5% of all new cancers in the USA and it remains the fifth most common [Citation1]. Over 75% are non-muscle-invasive bladder cancer (NMIBC) and of these, 50% will recur after the transurethral resection of bladder tumour (TURBT) [Citation2]. Caring for NMIBC involves continued surveillance, and frequently intravesical therapy. Intravesical therapy with chemotherapy and/or immunotherapy has suboptimal efficacy and some toxicity. The patients that are at especially high risk for a recurrence are found to be refractory to this form of therapy and often proceed to radical cystectomy [Citation3]. Muscle invasive bladder cancer (MIBC) often leads to a cystectomy as per guidelines and the associated significant morbidity and mortality [Citation4,Citation5]. Patients are faced with the anxiety and physical burden, not to mention that bladder cancer is the most expensive malignancy per patient [Citation6]. Novel therapies are being sought as ways to reduce the morbidities and costs of the disease, and potentially preserve that patient’s native bladder.
Pre-clinical data provide strong evidence that hyperthermia has the potential to induce anti-tumour immunity [Citation7]. There are at least five proposed mechanisms for the activation of the immune response by hyperthermia: (1) tumour cells increase surface expression of several markers (e.g. MHC class I) when exposed to heat, (2) heat causes the tumour to release HSPs, which in turn activates the host immune response, (3) heated tumour cells release exosomes that carry tumour antigens to the immune system, (4) heat alone directly activates the immune system, and (5) heat renders the tumour vasculature more permeable which allows for better trafficking of immune cells [Citation8].
Additionally, hyperthermia is an interesting topic of research because of the sensitising effect with chemotherapy and/or radiotherapy. Hyperthermia has been shown to enhance drug delivery and thermosensitises cancer cells to certain antineoplastic drugs [Citation9,Citation10]. In bladder cancer it leads to chemosensitisation by impairing the repair of damaged DNA and has synergistic action with cytotoxic agents, such as Mitomycin C [Citation11,Citation12].
Broadly, hyperthermia of the bladder can be achieved via different methods: radiofrequency emitting intravesical catheters (e.g. Synergo, operating at a frequency of 915 MHz [Citation10,Citation13,Citation14], externally heated chemotherapy fluid circulation in the bladder [Citation15], intravesical magnetic nanoparticles [Citation16], and external deep regional radiofrequency transmission with 70–110 MHz [Citation17]. While the intravesical heating methods are more commonly used and produce uniform temperatures across the urothelial lining of the bladder, there is a significant temperature gradient measured through increasing depth of the bladder wall. This temperature gradient is unlikely to be important for the treatment of NMIBC (which is confined to the urothelium and suburothelial lamina propria), but may have important consequences for the treatment of muscle invasive bladder tumours. Clinical evidence for the positive effect of radiofrequency-induced hyperthermia (operating at 915 MHz) was published in a meta-analysis in Ta-1 G1-3 NMIBC [Citation18]. After the combination of heat and intravesical therapy with Mitomycin C (MMC), a 59% relative reduction in NMIBC recurrence was seen compared to MMC alone. According to progression, lower rates were seen in case of chemohyperthermia (CH) although in most studies an adequate follow-up was lacking [Citation19].
Regional hyperthermia therapy (RHT) results in a more uniform temperature distribution across the bladder wall, and the field can be directed beyond the bladder itself to the lymphatic drainage. There are several commercially available RHT devices including the AMC device, BSD devices, and Thermotron device.
The BSD-2000 (BSD Medical, Salt Lake City, UT, USA) (and its predecessor, the BSD-1000) has been one of the most widely studied devices for RHT. It has been used in trials alone, and as an adjunct to surgery, radiation, and chemotherapy [Citation17,Citation20–23]. This system delivers RHT emitting electromagnetic radiofrequency radiation using a 100 ± 2 MHz radiofrequency Sigma Eye 24-array of dipole antennas that surround the patient as the applicator. Applicator selection is based on patient size. Proprietary software pre-plans treatment sessions. The bladder temperature can then be measured through the Foley catheter to ensure that the target temperature of 40–45 °C is achieved [Citation24]. The patient is then treated for a duration of 45–60 min once the therapeutic temperature is met. System power, frequency, relative antenna power, and phase were adjusted frequently during treatment to optimise bladder heating and patient tolerance based on measured temperatures, blood pressure, and patient verbal feedback.
The AMC 70-MHz phased array system consists of one or two rings, each with four wave guide antennas (aperture 33 × 21 cm) positioned around the pelvis of the patient and connected to a phase- and amplitude-controlled RF generator (SSB Electronic/Alba (Rome, Italy)). Patients are treated in supine position, using cooled water pillows to connect the antenna power to the patient. Phase settings are first optimised to create a focus at the rectum guided by an E-field probe in the rectum, followed by a phase shift of the dorsal and ventral antenna to move the focus from rectum to bladder [Citation25]. Final settings are validated by performing ΔT pulses for three phase settings with 40° phase shifts between the dorsal and ventral antenna [Citation26].
Thermometry is performed with 14 sensor thermocouple probes (Ella CS, Hradec Kralove, Czech Republic) inserted in catheters in the bladder, rectum (and vagina, if applicable). Temperature data are recorded using a 196-channel thermometry unit. Treatment continued for 60 min after bladder temperatures reached 41 °C, or for a maximum total duration of 90 min, whichever was shortest [Citation17,Citation27,Citation28].
The Thermotron (Yamamoto Vinita, Osaka, Japan) is an 8-MHz radiofrequency capacitive heating device. The output ranges from 271–965 W (578 ± 86.2 W). Large, deep seated tumours require large electrodes, and conversely small, superficial tumours respond to smaller electrodes. Pairs of electrodes with a cooling bolus are placed on the front and back of the patient. In patients with subcutaneous fat tissue >2 cm thickness, the superficial tissue was cooled for 15–20 min prior to and during heating with 10–15 °C saline. For heating deep areas of the body, an overlay bolus sheet in addition to the regular bolus was applied and the body surface was cooled continuously during heating [Citation29,Citation30].
This collaborative review provides a critical overview of current literature concerning the role of deep regional hyperthermia for the treatment of NMIBC and MIBC.
Evidence acquisition
The systematic review was registered with the PROSPERO database (registration number: CRD42015025780) and was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. A search of MEDLINE, Embase, Cochrane Library, CancerLit and ClinicalTrials.gov databases was undertaken for candidate manuscripts from 1966 through August 2015 and was not limited by language.
Candidate manuscripts were reviewed according to the Cochrane Collaboration criteria and reported following PRISMA guidelines.
Two reviewers (T.L. and A.G.) independently performed database searches, assessed candidate manuscripts for inclusion criteria, and extracted primary data. A detailed description of the search strategy is given in . Authors and experts in the field were asked for additional studies. In order to address a paucity of data on this topic, this systematic review followed the best-available-evidence approach and included several single-group studies, a decision made a priori [Citation31]. Reviewed studies consisted of prospective and retrospective cohorts in which all subjects received a single intervention and the outcome was assessed over time, but lacked an internal comparison.
Eligibility criteria
Studies were eligible if they met the following inclusion criteria: (1) adult patients with diagnosis of bladder cancer, (2) intervention with regional hyperthermia.
Studies were excluded if they involved: (1) paediatric patients, (2) animal studies, (3) review papers, (4) case reports, or (5) ablative temperatures >45 °C. The review was not restricted to the English language. Duplicate publications were also excluded and only manuscripts where the full text publication was retrievable were included.
Quality assessment
Study quality assessment was conducted using the Newcastle–Ottawa Quality Assessment Scale, which is used to evaluate studies based on three criteria: (1) patient selection, (2) comparability of groups, and (3) ascertainment of outcome for the Cochrane Risk of Bias Tool for clinical trials. Studies were assessed on a star scoring scale, with higher scores given for higher quality studies. Studies were not excluded from the review based on the perceived quality of the study. The primary end point was response. The secondary end points were recurrence, time to progression and adverse event (AE) rate.
Evidence synthesis
Search results
We identified 859 publications in the initial search, of which 24 met inclusion criteria for full text review. Of these, we were able to obtain data on the outcomes of interest for 15 publications. A total of 346 patients who underwent RHT treatment for bladder cancer were included. Characteristics of the studies which were included can be found in .
Table 1. Overview of all trials concerning regional hyperthermia therapy for bladder cancer.
Table 2. Overview of toxicity associated with regional hyperthermia therapy.
Non-muscle-invasive bladder cancer
There were two pilot studies using RHT with intravesical mitomycin C (MMC) in NMIBC [Citation21,Citation27], and a third trial using intravesical doxorubicin [Citation32]. Geijsen et al. [Citation27] employed the AMC 70 MHz device and involved six courses of RHT once a week, combined with intravesical chemotherapy with MMC in 18 patients with intermediate and high risk NMIBC after transurethral resection of the bladder tumour; it was then followed with a maintenance period over the next 12 months. During maintenance, the patients received a single course of RHT with MMC at 3, 6, 9, and 12 months [Citation27]. The primary end point of this trial was feasibility and toxicity. No grade 3 toxicity or higher was seen and the temperatures achieved in the bladder were satisfactory. They reported a recurrence-free survival rate of 78% at 24 months. However, 3/9 patients who had completed induction showed recurrent or progressive disease and one developed distant metastasis. 83% of the patients completed induction and 50% completed maintenance, or a 17% and 50% dropout rate, respectively.
Inman et al. [Citation21] assessed 15 NMIBC, in patients who were BCG refractory. The treatment protocol consisted of six courses of RHT once a week, combined with intravesical MMC. A maintenance schedule involved monthly RHT with intravesical MMC for four additional months. In total, 73% of patients completed induction and maintenance, with a dropout rate of 27%. Recurrence-free survival at 24 months was 33%, but this was maintained beyond 3 years. None of the recurrences progressed to muscle-invasive disease. Six of the ten patients who experienced recurrence underwent a cystectomy and all were node negative with no greater than T1 disease [Citation21]. The difference in length of the treatment period may have influenced the lower dropout rate and lower recurrence-free survival compared to Geijsen et al. [Citation27].
Ueda et al. [Citation32] performed a non-randomised trial comparing intravesical HPC-doxorubicin in 20 patients to intravesical HPC-doxorubicin combined with RHT using the Thermotron device in 11 patients with NMIBC. A complete response was noted in 6/11 (54.5%) patients with combination therapy versus 7/20 (35%) in intravesical therapy alone. A partial response was seen in 27.3% versus 30% in combination versus intravesical alone [Citation32]. The manuscript did not include results analysed by grade, a mean follow up, or a recurrence rate.
Additionally, there were three studies that looked at both NIMBC and MIBC [Citation20,Citation33,Citation34]. The efficacy and toxicity of these trials will be discussed below in the MIBC section.
Toxicity
Geijsen et al. determined toxicity would be a primary end point and used National Cancer Institute Common Terminology Criteria (CTCAE) 3.0 [Citation35]. Over 10% of patients experienced a grade 2 toxicity comprised of bladder complaints and lower back pain. No grade 3 or 4 toxicities were seen, but 6/18 patients discontinued therapy because of physical complaints, including MMC allergy. No damage was observed cystoscopically. The secondary objective was bladder cancer recurrence and progression. Similarly, Inman et al. [Citation21] did not report any grade 3 or higher toxicities. The most common adverse event was grade 1 urethral discomfort (40%) followed by abdominal discomfort from the device (33%) [Citation21]. Ueda et al. [Citation32] reported adverse events including dysuria and frequency. The intravesical alone group reported adverse effect rate of 25% and the cohort with RHT reported 27% [Citation32].
In conclusion, in NMIBC only limited phase I/II studies have been performed using regional hyperthermia in combination with different schedules of intravesical chemotherapy and different therapy regimens, making a real comparison difficult.
Muscle invasive bladder cancer
RHT has been used to treat MIBC alone and in combination with chemotherapy and radiation therapy. Early reports examined heterogeneous treatment protocols and vaguely defined categories of bladder cancer [Citation34,Citation36]. More dedicated trials examined each individual combination.
Radiohyperthermia
There has only been one prospective, randomised controlled trial of radiohyperthermia (RH) in MIBC as an adjunct to radiation therapy. Van der Zee et al. [Citation17] enrolled 358 patients with pelvic tumours, of whom 101 had MIBC. The patients were then randomised to radiotherapy alone (n = 49) or radiotherapy with hyperthermia (n = 52). A complete response was noted in 25/49 (51%) versus 38/52 (73%) in the radiotherapy versus combination groups respectively. The overall survival was 22% for radiotherapy and 28% for combination therapy at 3-year follow-up; this difference was not significant [Citation17].
There have been several observational cohorts examining a similar protocol of RH for a total of 63 patients. Masunaga et al. [Citation37] performed a clinical trial comparing radiotherapy in 21 patients to RH in 28 patients (6 NMIBC, 22 MIBC) using the Thermotron device prior to planned cystectomy [Citation37]. All patients received 24 Gy of radiation (4 Gy/day on 3 days/week) either alone, or in combination with hyperthermia. They then underwent a surgical resection within a week of completing this preoperative therapy. There were no local recurrences in the group treated with hyperthermia, while there was one instance in the radiotherapy alone group. Both groups experienced distant metastasis without a significant difference in numbers (two in radiotherapy alone, four in RH). Although the survival rate was higher in the RH group, they were unable to show statistical significance, probably due to the small number of patients. In another study in Japan, Uchibayashi et al. [Citation38] performed a trial with a cohort involving RH (mean 49 ± 3 Gy) in 19 patients. There was a complete response in 3/19 patients and a partial response in 8/19 patients. The survival period was 65, 31.5 and 11.6 months for T2, T3, T4 disease respectively [Citation38]. Sapozink et al. performed a pilot study with the BSD device on five bladder cancers [Citation23], and Nishimura et al. had a retrospective case series that applied RHT and radiation (40–50 Gy) to four bladder tumours [Citation39]. There were five complete responses among these patients without mention of the tumour stage. Noguchi et al. [Citation33] compared a cohort of patients with RH to another cohort of RH (40 Gy) plus chemotherapy (RH + MVAC). The response rates between the groups were similar: a complete response of 4/17 and 4/18 and a partial response of 5/17 and 7/18 for RH and RH + MVAC respectively [Citation33]. Petrovich et al. [Citation22] examined 20 patients with urothelial cell that were described as advanced tumours. All patients received hyperthermia and this may have been alone or with chemotherapy or with radiotherapy. A complete response was not seen in any of the patients and only two partial responses were noted. Both of the partial responses had received radiation in addition to hyperthermia [Citation22].
Toxicity
In Noguchi et al. [Citation33] both the RH and RH + MVAC groups two thirds experienced tenesmus and frequency. Fewer than 20% experienced fatty necrosis. They did report one case of a contracted bladder thought to be related to the hyperthermia but did not distinguish side effects by tumour type. Sapozink et al. did not distinguish amongst histology, but did note that the most common toxicities were pain and bladder spasm [Citation23]. The RCT listed adverse events without a grade or distinction to tumour, but included subcutaneous burns in 20 patients, skin burns in five patients, and urinary tract infection [Citation17].
Chemohyperthermia
There have been two publications looking specifically at RHT with intravenous chemotherapy. Rietbroek et al. [Citation28] employed the AMC 70 MHz device at weekly intervals with cisplatin for four patients with T3 or T4 disease for a minimum of four cycles. Two patients had partial response with a duration of 5 and 7 months [Citation28]. Uchibayashi et al. [Citation38] performed a trial with two cohorts of MIBC; the CH cohort consisted of 19 patients receiving THP-adriamycin and nine patients received platinum-based chemotherapy. The other cohort involved RH and will be discussed below. There was a complete response in 2/27 patients and a partial response of 7/27 patients. The survival period in those treated with THP was 45.2, 15.4, and 13.5 months for T2, T3, T4 disease respectively, and for cisplatin 24, 36, and 8.5 months [Citation38].
Toxicity
Rietbroek et al. [Citation28] evaluated toxicity using the World Health Organization grading system, but did not report toxicities by tumour type. They did not report any grade 4 toxicities, and felt that the only hyperthermia related toxicities were fat necrosis (11%), two cases of skin burns, and most commonly, mild pain (31%) [Citation28]. Uchibayashi et al. reported burns in 13/46 patients and anorexia in 11 patients [Citation38]. Measured bladder temperatures were not given in relation to these toxicities.
Quadrimodal therapy
Several studies have looked at the combination termed ‘quadrimodal’ therapy, defined as surgery (transurethral resection of the bladder tumour), chemotherapy, radiation therapy, and RHT. Ohguri et al. used this therapy in order to preserve the bladders of three patients with MIBC (all T2 disease, one N0, 2 N1) [Citation40]. This protocol involved higher temperatures (average from 44.5°, 47°, 47.4 °C) than is typically achieved in the majority of trials, but was chosen for better local response. The hyperthermia was given immediately following radiotherapy (total dose 66–70 Gy) during the course of chemotherapy. All three patients achieved a complete response without any recorded recurrence or metastasis (16, 18, and 40 months).
As mentioned earlier, Wittlinger et al. [Citation20] examined quadrimodal therapy in MIBC. Their protocol was more defined than the cases described by Ohguri et al. [Citation40] and involved 19 patients with T2 MIBC in addition to 26 high risk NMIBC patients. All patients underwent TURBT. In the entire trial 15/45 patients completed less than the minimum number of RHT sessions [Citation20]. At the initial re-TURBT, 96% of patients had a complete response and recurrence-free survival was 81% at three years. There were five disease-specific deaths, all of which involved distant metastases with complete local control. Disease-specific survival was 88% at 3 years and overall survival was 80% at 3 years while disease-free survival and metastasis-free survival were 71% and 89% respectively. The 3-year bladder preservation rate was 97% for those alive, and 96% overall.
Toxicity
In Ohguri et al. no grade 3 or greater toxicity was reported, but all patients experienced cystitis [Citation40]. Wittlinger et al. recorded toxicity using CTCAE, version 3.0 [Citation20,Citation35]. Grade 3 and 4 sequelae were reported in 24% of patients: specifically, one salvage cystectomy for haematuria, one contracted bladder (<100), a reduced bladder capacity (100–200 mL) was seen in 13% of patients, and two bowel obstructions [Citation20].
Limitations
This systematic review represents a comprehensive summary of a clinically relevant topic. The inclusion of single-group studies suffers from several drawbacks. The lack of internal comparisons limits the role for inference of the comparative effectiveness, and alternative explanations should be considered for the effects seen other than the intervention being studied. However, single-arm reviews are appropriate for the identification and possible quantification of harms. They are common in the evaluation of novel technology and compose the majority of available data. The inclusion of such studies is common amongst systematic reviews and criteria for inclusion have been proposed by Ip et al. [Citation41]. The choice of a proxy comparison is open to bias because it depends upon assumptions. For many of the studies without an internal control, the unlikelihood of spontaneous regression of disease coupled with the documented response rates is informative. Comparisons of adverse events are difficult because the older studies used non-validated questionnaires, whereas more recent studies used CTCAE. There is also inconsistent analysis amongst the trials. The populations examined have heterogeneity in demographic factors (particularly race and functional status), and span a large period of time. Similarly, there was no standardised methodology.
Conclusions
Our systematic review suggests that with regional hyperthermia high bladder temperatures can be achieved with acceptable toxicity. It may be effective in both the NMIBC and MIBC setting regardless of adjunctive therapies. It offers improved efficacy at tumour eradication, reduced recurrences, and the potential to spare the native bladder. This systematic review serves as a call to action for better designed clinical trials, and indeed, there are 109 ongoing clinical trials involving some form of hyperthermia [Citation42]. The promise of deep regional hyperthermia seen by the effect size has been largely ignored, secondary to the disparate and small body of evidence. This nascent technology has been smouldering for years, and is now ready to be delivered to the forefront of therapy, particularly for high risk NMIBC, and for those patients not suitable for a cystectomy.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ajay Gopalakrishna was funded by the NIH grant TL1TR001116-03.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29.
- Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013;63:234–41.
- Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette–Guerin. Eur Urol 2015.
- The National Comprehensive Cancer Network. Bladder Cancer, version 2.2015.
- Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol 2007;178:2314–30.
- Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics 2003;21:1315–30.
- Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 2012;28:528–42.
- Toraya-Brown S, Fiering S. Local tumour hyperthermia as immunotherapy for metastatic cancer. Int J Hyperthermia 2014;30:531–9.
- Engelhardt R. Rational for clinical application of hyperthermia and drugs. Rev Med Chir Soc Med Nat Iasi 1987;91:347–52.
- Gofrit ON, Shapiro A, Pode D, Sidi A, Nativ O, Leib Z, et al. Combined local bladder hyperthermia and intravesical chemotherapy for the treatment of high-grade superficial bladder cancer. Urology 2004;63:466–71.
- Wallner KE, Banda M, Li GC. Hyperthermic enhancement of cell killing by mitomycin C in mitomycin C-resistant Chinese hamster ovary cells. Cancer Res 1987;47:1308–12.
- van der Heijden AG, Jansen CF, Verhaegh G, O'Donnell M A, Schalken JA, Witjes JA. The effect of hyperthermia on mitomycin-C induced cytotoxicity in four human bladder cancer cell lines. Eur Urol 2004;46:670–4.
- Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int 2011;107:912–18.
- Rigatti P, Lev A, Colombo R. Combined intravesical chemotherapy with mitomycin C and local bladder microwave-induced hyperthermia as a preoperative therapy for superficial bladder tumors. A preliminary clinical study. Eur Urol 1991;20:204–10.
- Sousa A, Inman BA, Pineiro I, Monserrat V, Perez A, Aparici V, et al. A clinical trial of neoadjuvant hyperthermic intravesical chemotherapy (HIVEC) for treating intermediate and high-risk non-muscle invasive bladder cancer. Int J Hyperthermia 2014;30:166–70.
- Oliveira TR, Stauffer PR, Lee CT, Landon CD, Etienne W, Ashcraft KA, et al. Magnetic fluid hyperthermia for bladder cancer: A preclinical dosimetry study. Int J Hyperthermia 2013;29:835–44.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000;355(9210):1119–25.
- Lammers RJ, Witjes JA, Inman BA, Leibovitch I, Laufer M, Nativ O, et al. The role of a combined regimen with intravesical chemotherapy and hyperthermia in the management of non-muscle-invasive bladder cancer: A systematic review. Eur Urol 2011;60:81–93.
- Yuan Y, Cheng KS, Craciunescu OI, Stauffer PR, Maccarini PF, Arunachalam K, et al. Utility of treatment planning for thermochemotherapy treatment of nonmuscle invasive bladder carcinoma. Med Phys 2012;39:1170–81.
- Wittlinger M, Rodel CM, Weiss C, Krause SF, Kuhn R, Fietkau R, et al. Quadrimodal treatment of high-risk T1 and T2 bladder cancer: Transurethral tumor resection followed by concurrent radiochemotherapy and regional deep hyperthermia. Radiother Oncol 2009;93:358–63.
- Inman BA, Stauffer PR, Craciunescu OA, Maccarini PF, Dewhirst MW, Vujaskovic Z. A pilot clinical trial of intravesical mitomycin-C and external deep pelvic hyperthermia for non-muscle-invasive bladder cancer. Int J Hyperthermia 2014;30:171–5.
- Petrovich Z, Emami B, Kapp D, Sapozink MD, Langholz B, Oleson J, et al. Regional hyperthermia in patients with recurrent genitourinary cancer. Am J Clin Oncol 1991;14:472–7.
- Sapozink MD, Gibbs FA, Jr., Egger MJ, Stewart JR. Regional hyperthermia for clinically advanced deep-seated pelvic malignancy. Am J Clin Oncol 1986;9:162–9.
- Juang T, Stauffer PR, Craciunescu OA, Maccarini PF, Yuan Y, Das SK, et al. Thermal dosimetry characteristics of deep regional heating of non-muscle invasive bladder cancer. Int J Hyperthermia 2014;30:176–83.
- Crezee J, Van Haaren PM, Westendorp H, De Greef M, Kok HP, Wiersma J, et al. Improving locoregional hyperthermia delivery using the 3-D controlled AMC-8 phased array hyperthermia system: A preclinical study. Int J Hyperthermia 2009;25:581–92.
- Kok HP, Ciampa S, de Kroon-Oldenhof R, Steggerda-Carvalho EJ, van Stam G, Zum Vorde Sive Vording PJ, et al. Toward online adaptive hyperthermia treatment planning: Correlation between measured and simulated specific absorption rate changes caused by phase steering in patients. Int J Radiat Oncol Biol Phys 2014;90:438–45.
- Geijsen ED, de Reijke TM, Koning CC, Zum Vorde Sive Vording PJ, de la Rosette JJ, Rasch CR, et al. Combining mitomycin C and regional 70 MHz hyperthermia in patients with nonmuscle invasive bladder cancer: A pilot study. J Urol 2015;194:1202–8.
- Rietbroek RC, Bakker PJ, Schilthuis MS, Postma AJ, Zum Vorde Sive Vording PJ, Gonzalez Gonzalez D, et al. Feasibility, toxicity, and preliminary results of weekly loco-regional hyperthermia and cisplatin in patients with previously irradiated recurrent cervical carcinoma or locally advanced bladder cancer. Int J Radiat Oncol Biol Phys 1996;34:887–93.
- Lee CK, Song CW, Rhee JG, Foy JA, Levitt SH. Clinical experience using 8 MHz radiofrequency capacitive hyperthermia in combination with radiotherapy: Results of a phase I/II study. Int J Radiat Oncol Biol Phys 1995;32:733–45.
- Hiraoka M, Jo S, Akuta K, Nishimura Y, Nagata Y, Takahashi M, et al. Clinical results of radiofrequency capacitive hyperthermia in deep-seated tumors. Gan No Rinsho Jap J Cancer Clin 1986;32:1679–84.
- Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: What it is and what it isn't. 1996. Clin Orthopaed Rel Res 2007;455:3–5.
- Ueda K, Sakagami H, Masui Y, Okamura T. Single instillation of hydroxypropylcellulose-doxorubicin as treatment for superficial bladder carcinoma. Cancer Chemother Pharmacol 1994;35:S81–S3.
- Noguchi S, Kubota Y, Miura T, Shuin T, Hosaka M. Use of methotrexate, vinblastine, adriamycin, and cisplatin in combination with radiation and hyperthermia as neo-adjuvant therapy for bladder cancer. Cancer Chemother Pharmacol 1992;30:S63–S5.
- Hisazumi H, Nakajima K. Eight-MHz RF hyperthermia in urological malignancies. Gan To Kagaku Ryoho Cancer Chemother 1988;15:1382–6.
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81.
- Naito K, Hasegawa T, Ishida T, Yamamoto H, Mihara S, Komatsu K, et al. A clinical survey of advanced bladder cancer: Treatment of advanced and non-resectable bladder cancer. Hinyokika Kiyo Acta Urol Jap 1991;37:1601–6.
- Masunaga SI, Hiraoka M, Akuta K, Nishimura Y, Nagata Y, Jo S, et al. Phase I/II trial of preoperative thermoradiotherapy in the treatment of urinary bladder cancer. Int J Hyperthermia 1994;10:31–40.
- Uchibayashi T, Yamamoto H, Kunimi K, Koshida K, Nakajima K. Radiofrequency capacitive hyperthermia combined with irradiation or chemotherapy for patients with invasive bladder cancers. Int Urol Nephrol 1995;27:735–41.
- Nishimura Y, Hiraoka M, Jo S, Akuta K, Nagata Y, Masunaga S, et al. Radiofrequency (RF) capacitive hyperthermia combined with radiotherapy in the treatment of abdominal and pelvic deep-seated tumors. Radiother Oncol 1989;16:139–49.
- Ohguri T, Imada H, Nomoto S, Kato F, Yahara K, Morioka T, et al. Initial experience of bladder preservation therapy using chemoradiotherapy with regional hyperthermia for muscle-invasive bladder cancer. Thermal Med 2005;21:151–8.
- Ip S, Paulus JK, Balk EM, Dahabreh IJ, Avendano EE, Lau J. Role of Single Group Studies in Agency for Healthcare Research and Quality Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality, 2013.
- Cihoric N, Tsikkinis A, van Rhoon G, Crezee H, Aebersold DM, Bodis S, et al. Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia 2015;31:609–14.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. PLoS Med 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. Epub 2009 Jul 21.