Abstract
Purpose: The purpose of the study was to prospectively evaluate the safety and the efficacy of percutaneous radio-frequency ablation of hepatocellular carcinoma adjacent to the gastrointestinal tract.
Materials and methods: From April 2012 to November 2015, 141 hepatocellular carcinoma nodules that underwent ultrasound-guided percutaneous radio-frequency ablation were included. A total of 52 lesions were located less than 5 mm from the gastrointestinal tract in the study group, and 89 lesions were located more than 5 mm from hepatic surface in the control group. Ethanol (2.5–9.6 mL) was injected into marginal tissue of tumour in five lesions of the study group. During the ablation, the temperature of marginal ablation tissue proximal to the gastrointestinal tract was monitored and controlled at 45–56 °C for more than 10 min in the study group. We compared the results of ablation between the two groups.
Results: In total 48 of 52 tumours (92.3%) in the study group and 84 of 89 tumours (94.4%) in the control group achieved complete ablation (P = 0.63). Local tumour progression was found in eight tumours (15.4%) in the study group and 11 tumours (12.4%) in the control group during follow-up (P = 0.61). There were neither immediate nor peri-procedural major complications in both groups, grade I (Clavien–Dindo classification). One case developed biloma at 5-month follow-up in the study group, Clavien–Dindo gradeIII.
Conclusions: Percutaneous radio-frequency ablation is safe and achieves a high complete ablation rate for the treatment of hepatocellular carcinoma adjacent to the gastrointestinal tract.
Introduction
Hepatocellular carcinoma (HCC) ranks as the fifth most common malignant tumour globally, accounting for the third most common cause of cancer-related death [Citation1]. Its incidence has been increasing worldwide due to the spread of hepatitis B and C virus (HBV and HCV) infection [Citation2]. Most patients with HCC confined to the liver are not candidates for resection because of the frequent association with cirrhosis and other contraindications such as impaired liver function or multiplicity of lesions [Citation3–4]. Furthermore, surgical resection is associated with a recurrence rate of 40–60% [Citation5]. Although orthotopic liver transplantation offers the chance for therapeutic success, its performance is limited by a shortage of donor organs [Citation6]. Moreover, HBV or HCV infection recurs after transplantation, leading to severe liver damage [Citation7].
For the past few years, non-surgical treatment modalities have been developed in treating primary and metastatic hepatic tumours [Citation8–9]. Image-guided thermal ablation using different energy sources, such as radio-frequency (RF), microwave (MW), cryoablation, high intensity focused ultrasound (HIFU) or laser has been proven to play an important role against hepatic malignant tumours [Citation10–17]. The benefits of thermal ablation include low morbidity, few complications and repeatability for recurrence [Citation18–20].
Although image-guided thermal ablation has been considered a safe technique, a broad spectrum of complications has been reported in several large series [Citation21–24]. Among them, the most important major complication due to thermal damage was perforation of the gastrointestinal tract, which has been reported as a serious complication of thermal ablation occurring with an overall incidence of 0.1–0.3% [Citation25–28]. Some authors have recommended that percutaneous thermal ablation should be avoided when treating liver tumours adjacent to the gastrointestinal tract [Citation25]. Some authors reported that for treating such tumours, special interventional techniques such as introducing artificial ascites and hyaluronic acid gel injection are necessary to separate the gastrointestinal tract from the liver [Citation29–33]. However, there are certain factors that result in poor visibility of the puncture needle on ultrasound (US), which increases the difficulty of insertion and the occurrence of complications such as intraperitoneal bleeding, intestinal perforation and peritoneal seeding [Citation30]. These factors include a history of laparotomy, interference from gastrointestinal gas, and a lack of operation experience. The interposition of a balloon catheter is another method that serves to increase the distance between the liver and the adjacent gastrointestinal tract [Citation34], but this technique is rarely used. To our knowledge, no research on a relatively large sample concerning thermal ablation with the technique has been reported.
Thermal injury may be prevented by strict temperature monitoring of hepatic tissue abutting vital organs or structures. Our previous in vivo experimental study showed that thermal ablation for liver tissue adjacent to gastrointestinal tract is safe by monitoring the temperature of the ablation area fluctuating between 55–65 °C for 6 min [Citation35].
This prospective study was undertaken to assess the safety and efficacy of RF ablation under temperature monitoring for treatment HCC adjacent to the gastrointestinal tract.
Materials and methods
Study population
From April 2012 to November 2015, 261 patients with HCC underwent percutaneous radiofrequency (RF) ablation with curative intention at the authors’ institutions. Indication criteria for percutaneous RF ablation were unresectable lesions or patient refusal to undergo surgery, tumour accessible via a percutaneous approach, single lesion of 5.0 cm or smaller, three or fewer multiple lesions with a maximum dimension of 3.0 cm or less in each lesion, absence of portal vein thrombosis or extrahepatic metastases, prothrombin time of less than 25 s, prothrombin activity higher than 40%, and platelet count higher than 40 cells ×109/L. In total 52 patients with 52 hepatic lesions adjacent to the gastrointestinal tract, distance less than 5 mm were included in the study group, and 83 patients with 89 hepatic lesions, distance more than 5 mm from the hepatic surface were included in control group. There were 33 men and 19 women in the study group, 57 men and 26 women in the control group. Age range was 31–79 years (mean 54.7 ± 11.5 years) in the study group and 33–80 years (mean 57.2 ± 9.8 years) in the control group. The maximum diameter of lesions ranged from 1.0 to 4.8 cm (mean 2.5 ± 1.2 cm) in the study group and from 0.9 to 4.9 cm (mean 2.6 ± 1.0 cm) in the control group. For these 52 lesions in the study group, 21 abutted the stomach, 18 abutted the colon and 13 abutted the small bowel.
Liver function was determined using the Child-Pugh classification and model for end-stage liver disease (MELD) score. Child A and Child B patients were considered suitable candidates, whereas Child C status represented a contraindication for RF ablation. There were 41 patients in the study group and 62 patients in the control group with Child A disease. The mean MELD score was 8.7 (range 5–12) in the study group and 9.1 (range 5–14) in the control group.
There was no significant difference in clinical backgrounds between the study group and control group ().
Table 1. Baseline characteristics of patients.
This investigation was approved by our institutional review board. Written informed consent was obtained from all patients.
Pre-ablation work-up and histological diagnosis
Pretreatment investigation included US, contrast-enhanced US (CEUS), contrast-enhanced CT and/or contrast-enhanced MRI, and tumour marker assay in all patients. The maximum diameter of the index tumours were measured on CEUS. US and CEUS were performed using the Acuson Sequoia 512 system (Mountain View, CA) with 3.5–5.0 MHz curved-array multifrequency transducers. The Acuson 512 was equipped with software for contrast media (contrast pulse sequences, or CPS). The US contrast agent was Sonovue, sulphur hexafluoride microbubbles for injection (Bracco, Milan, Italy). The shortest distance from the edge of the lesion to the gastrointestinal tract was confirmed by contrast enhanced CT or MRI in the transverse plane and coronal plane.
Histological diagnosis was obtained by US-guided tumour biopsy using an 18-gauge needle (Bard Medical, Covington, GA, USA) in all patients. In patients with multiple nodules, at least one biopsy was performed. If new tumours emerged after ablation, biopsies of the new nodules were performed.
Laboratory data
Since an increase in serum alpha-fetoprotein (AFP) levels may indicate recurrence or new nodules, AFP assay was performed in all patients before and after RF ablation. The AFP level was abnormal (range 29–9100 μg/L) in 33 patients in the study group and 59 patients in the control group. The AFP level was normal (≤20 μg/L) in the remaining 43 patients. Serum AFP assay was performed at 1 month after treatment, and follow-up was performed at an interval of 3 months.
RF ablation procedure
All treatments were performed in the operating room under intravenous anaesthesia. The RF system (Celon Lab Power, Olympus, Hamburg, Germany) provides a maximum power output of 250 W (rated frequency, 470 ± 10 kHz) and is capable of connecting one to three electrodes with an exposed tip of 20–40 mm. Each electrode has a 20-cm shaft (diameter of 1.8 mm) which could be easily visualised on sonographic imaging. The system consists of three water-pumping machines, which can drive three cool-tip needle electrodes. A detailed protocol was worked out for each patient on an individual basis before treatment, which included the placement of the electrodes, power output setting, emission time, and appropriate approach. In general, for tumours less than 1.5 cm in diameter, a single electrode was used; for tumoirs 1.5 cm or larger, multiple electrodes were required. An output setting between 40 W and 60 W was used during ablations. During the therapy we monitored the hyperechoic area of ablation using real-time US and the thermal monitoring to decide the end point of treatment. After ablation the electrodes were slowly withdrawn and RF emission was continued until the electrodes were pulled just under the skin entrance site. This method allowed needle track cauterisation to prevent tumour seeding and to minimise bleeding after ablation. All therapy was performed by experienced radiologists with more than 10 years of experience with interventional procedures. After ablation every patient received CEUS immediately to evaluate whether the ablation area had covered the tumour. If residual tumour was detected, additional treatment was performed.
Thermal monitoring procedure
A thermal monitoring system attached to the RF unit was used during treatment for the study group. With US guidance, one or two 21-gauge tissue thermal monitoring needles (Kangyou Medical, Nanjing, China) were placed into the marginal tissue of the tumour or liver proximal to the gastrointestinal tract for real-time temperature monitoring during the ablation to protect the gastrointestinal tract from thermally mediated injury (). The mean distance between the RF ablation electrode and thermal monitoring needle was 10.2 ± 1.1 mm (range 9.5–11.5 mm). Based on our experimental evidence and clinical experience, the temperature cut-off point for ablation therapy was set at 56 °C in the patients. If the measured temperature reached 56 °C, emission of RF was stopped immediately, and was activated again once the temperature had decreased to 45 °C. By the end of the treatment session the measured temperature fluctuated between 45 and 56 °C for more than 10 min (640–1660 s) and did not exceed 54 °C for more than 3 min in the patients ().
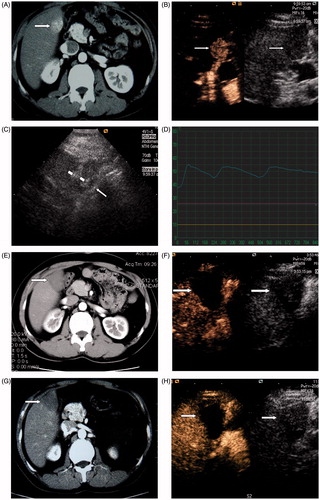
Figure 1. A 45-year-old man with HCC in the right lobe of the liver treated with RF ablation combined with PEI. (A) Before ablation, contrast enhanced CT scans shows a 2.3-cm tumour located at the edge of hepatic segment V adjacent to the hepatic flexure of colon (arrowhead). (B) Before ablation, CEUS shows the tumour hyper-enhanced in the arterial phase. (C) Two RF ablation needles (short arrowhead) were placed into the tumour and one thermal monitoring needle (long arrowhead) was placed into the margin of the tumour proximal to the hepatic flexure of the colon for real-time temperature monitoring during the procedure. (D) The temperature monitoring curve during RF ablation for a tumour adjacent to the gastrointestinal tract. The temperature of the marginal tumor tissue adjacent to the gastrointestinal tract was monitored and controlled to fluctuate between 45 °C and 56 °C during the whole treatment procedure. (E) Contrast enhanced CT 3 months after treatment shows the tumour (arrowhead) is completely ablated. (F) CEUS 3 months after treatment shows the tumour is completely ablated. (G), Contrast enhanced CT scan 18 months after treatment shows the ablation zone (arrowhead) had no enhancement. (H) CEUS 18 months after treatment shows the ablation zone (arrowhead) had no enhancement.
Percutaneous ethanol injection (PEI)
Among 52 patients in the study group, five patients in the study group had a history of hepatic resection. For those five patients, PEI was adopted as the adjuvant therapy before RF ablation. All ethanol injections were planned beforehand to ensure that patients with no history of allergy to ethanol. The standard total dose of injected ethanol was calculated according to the tumour’s size. The diffusion of ethanol in the tumour was monitored under real-time US guidance; if we found the hyperechoic zone completely covered the tumour margin adjacent to the gastrointestinal tract, we stopped injection and then slowly withdrew the needle. In particular, the injection was also stopped if we found significant ethanol leaked into surrounding veins or surrounding structures. With US guidance, one to two 21-gauge percutaneous ethanol injection needles (Hakko, Tokyo, Japan) were placed into marginal tumour tissue proximal to the gastrointestinal tract for six tumours in the study group. There are three side holes at the point of the needle, which allow uniform distribution of the injected ethanol. Dehydrated, sterile, 99.5% ethanol was slowly injected into marginal tissue of tumour under US guidance before RF ablation, with 1-3 injection sessions (mean 1.3 ± 0.6 sessions) and total dose of 2.5–8.6 mL (mean 4.4 ± 2.1 mL) by the end of the treatment.
Complications evaluation and follow-up
The Clavien–Dindo classification was used to evaluate complications after treatment [Citation36]. The follow-up period was calculated starting from the beginning of RF ablation for all patients. Therapeutic efficacy was assessed on the basis of an integrative evaluation of contrast-enhanced imaging and AFP levels. Contrast-enhanced CT or MRI and CEUS were repeated at 1 month and at 3 months within 1 year after RF ablation treatment and then at 6-month intervals after treatment. The median follow-up time was 17 months (range 1–38 months) in the study group and 18.5 months (range 3–39 months) in the control group.
Statistical analysis
Data analysis was done using SPSS software for windows (Version 13.0, SPSS, Chicago, IL). Measurement data are presented as mean ± standard deviation (SD). Continuous variable were compared between the subsets using Student’s t-test. Fisher’s exact test was applied to compare categorical variables between the subsets. Chi-square test was undertaken to compare the proportions. A difference with a P value of less than 0.05 was considered statistically significant.
Results
Outcome of RF ablation
All patients were treated successfully. Forty-eight of 52 (92.3%) tumours in the study group and 84 of 89 (94.4%) tumours in the control group achieved complete ablation as confirmed at 1-month follow-up contrast-enhanced imaging (P = 0.63). Among the tumours with more than 6 months follow-up after treatment, 33 of 37 (89.2%) in the study group and 66 of 71 tumours (93.0%) in the control group were completely ablated (P = 0.50) (). No significant statistical difference in the rate of complete ablation was found between the two groups. Total treatment duration for one lesion was 1045–2160 s in the study group and 860–1750 s in the control group. Although the size of tumours in the study group had no significant difference to those in the control group, lesions in the study group required longer duration of treatment than those in the control group; however, they did not require a larger number of treatment sessions ().
Table 2. Comparison of therapeutic data in the study an control group.
Changes in AFP level
Among the 33 patients with abnormal AFP level in the study group, 21 (63.6%) had a completely negative result, and 10 (30.3%) had the level decrease by more than 50% 1 month after the ablation. Among the 59 patients with abnormal AFP levels in the control group, 37 (62.7%) had a completely negative result, and 15 (25.4%) had the level decrease by more than 50% 1 month after the ablation. There was no significant difference in the rate of AFP level decrease between the two groups (P = 0.74).
Complications
There were neither immediate nor peri-procedural major complications in either study and control groups, Clavien–Dindo grade I . In the study group, four cases suffered mild or moderate abdominal pain, three cases developed nausea and vomiting, and one case developed a small amount of pleural effusion after ablation. In the control group, seven cases suffered mild abdominal pain, five cases developed a small amount of pleural effusion, and two cases developed a fever with temperature >39 °C. All the symptoms self-relieved in 3–7 days. There was no significant difference in the incidence of complications between the two groups (P = 0.82). In particular, there was no gastrointestinal perforation in the study group. One patient in the study group experienced major complication related to the RF ablation procedure, Clavien–Dindo grade III. The asymptomatic patient developed biloma at 5-month follow-up MRI and was treated with antibiotics and percutaneous catheter drainage under US guidance.
Local tumour progression
Local tumour progression was found in 8 of 52 tumours (15.4%) in the study group and in 11 of 89 tumours (12.4%) in the control group by follow-up contrast-enhanced imaging (P = 0.61). The eight local recurrence tumours in the study group abutted the stomach (one), colon (five) and small bowel (two). Among the eight patients, four underwent repeated RF ablation, two underwent hepatic resection, and two were treated with TACE. The maximum diameters of the local recurrence tumours were 1.5–4.2 cm (mean 2.9 ± 1.0 cm). There was no significant statistical difference in the size of tumour between completely ablated tumours (range 1.0–4.6 cm, mean 2.7 ± 0.9 cm) and local tumour progression tumours in the study group (P = 0.83).
Discussion
RF ablation has been reported to be an effective technique for treating malignant hepatic tumours [Citation12,Citation21–23]. The recent development of novel techniques and instruments has significantly improved the therapeutic effects of RF ablation for medium to large tumours (3–6 cm) [Citation37–38]. In fact, tumours are often found in difficult locations such as adjacent to the gallbladder, diaphragm, large vessels and gastrointestinal tract. These structures and organs may be injured during the treatment, resulting in subsequent complications including perforation of the gastrointestinal tract, which has been reported as a serious complication of thermal ablation [Citation39]. In addition, RF ablation with an insufficient safety margin of 0.5 cm or greater might result in residual tumour and recurrence, restricting its application [Citation40–41]. At present, thermal ablation assisted with artificial ascites is a well-known technique for treating tumours adjacent to the gastrointestinal tract, but haemorrhage and tumour seeding are potential complications related to artificial ascites because the ascites can wash away coagulation substances at the puncture site and decrease the compression on the opposing abdominal wall against the liver, facilitating the dissemination of tumour cells at the same time [Citation30]. In addition, peri-hepatic adhesion due to a prior history of hepatic resection or TACE and a prominent omentum surrounding the hepatic capsule can cause technique failure for successful induction of artificial ascites [Citation33].
The major concern for thermal ablation for tumours adjacent to the gastrointestinal tract other than those for tumours in other sites was gastrointestinal perforation. The main factor for thermal damage in tissue is temperature over the threshold of coagulation. Thus we monitored the temperature of the marginal tissue of tumour or liver proximal to the gastrointestinal tract to avoid thermal damage of the adjacent bowel loop and also to ensure treatment efficacy of the marginal tumour tissue. Based on the threshold temperature of coagulation necrosis [Citation42] and our previous in vivo experimental study, we intended to control the temperature of marginal tumour tissue to lower than 56 °C, the threshold temperature of coagulation, to avoid thermal damage of the adjacent bowel loop, but higher than 45 °C to ensure the thermal field covered the marginal field of the tumour. During the treatment procedure we observed that bowel peristalsis became more active. This normal physiological action helped to avoid sustaining heating of the same area. It is worth noting that for patients with abdominal surgery history the adhesion between liver and gastrointestinal tract may decrease bowel peristalsis and the risk of thermal injury to the bowel loop would be increased. Our results, with no immediate and peri-procedural complications, show our modality is feasible and effective for the treatment of HCC adjacent to the gastrointestinal tract.
In our study we found no significant difference either in the complete ablation rate or in the local tumour progression rate between the study group and control group. Although the size of tumours in the study group showed no significant difference from that in the control group, tumours in the study group required a longer duration of ablation than those in the control group because of intermittent emission of radio-frequency electrodes to avoid thermal damage to adjacent bowel loops. The results of the study were similar to previous reports of thermal ablation for such tumours with other adjuvant techniques, such as artificial ascites and hyaluronic acid gel injection between the tumour and gastrointestinal tract [Citation30,Citation32–33]. However, the number of treatment sessions was not increased in our study, which profited from detailed treatment protocol design and accurate placement of electrodes, tissue thermal monitoring needles and ethanol injection needles.
As RF ablation is a first-line treatment of major local ablative techniques for the treatment of HCC, PEI therapy remains useful in select settings, particularly for the ablation of tumours whose locations put them at risk with other thermo-ablative modalities. In our study we added adjuvant therapy to a low dose of ethanol injection in the vicinity of the adjacent gastrointestinal tract in five patients who had a history of hepatic resection, to achieve complete necrosis of the marginal tissue of tumour. PEI has two effects: procuring chemical ablation for the marginal cells of the tumour and achieving a synergistic effect with combined use of ethanol and thermal ablation. Experimental and clinical reports have shown that combined use of ethanol and radio-frequency or microwave ablation causes a synergistic necrotising effect, with coagulation volumes clearly larger than those usually obtained with RF ablation, MW ablation or PEI alone [Citation43–45].
There were neither immediate nor peri-procedural complications in either study and control groups. One case in the study group developed biloma related to the procedure at 5-month follow-up, and the rate of major complications in this study was comparable with the rate reported in large studies of patients with liver tumours treated with conventional percutaneous RF ablation or use of artificial ascites [Citation22,Citation33]. There was no collateral thermal injury such as perforation of the gastrointestinal wall. We regard the RF ablation under the strict temperature monitoring as the safe and effective technique in percutaneous thermal ablation of hepatic tumour adjacent to the gastrointestinal tract.
This study also has some limitations. First, these data were obtained from only two centres at which there was much experience with percutaneous thermal ablation procedures. Therefore, higher complete ablation rates and lower local tumour progression rates were achieved. A further multicentre study with a larger number of patients and a prolonged observation time is required. Second, at present, a thermal monitor can only provide one temperature point. The study of temperature monitoring for multiple points with the thermal monitoring system may be more precise for the evaluation of ablation effect in the future. Third, this study was our experience with RF ablation, it may need to be further confirmed whether it could be used in other thermal ablation settings.
Conclusion
In conclusion, under strict temperature monitoring, percuteneous RF ablation is feasible and effective for treating HCC adjacent to the gastrointestinal tract. This modality may provide a new way for treating HCC adjacent to the gastrointestinal tract.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: A global and regional perspective. Oncologist 2010;15(Suppl 4):5–13.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362(9399):1907–17.
- Mazzaferro V, Chun YS, Poon RT, Schwartz ME, Yao FY, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg Oncol 2008;15:1001–7.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: Long-term results of treatment and prognostic factors. Ann Surg 1999; 229:216–22.
- Kulik LM, Chokechanachaisakul A. Evaluation and management of hepatocellular carcinoma. Clin Liver Dis 2015;19:23–43.
- Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology 2002;122:889–96.
- Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: From percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology 2002;62:S64–S8.
- Haemmerich D, Laeseke PF. Thermal tumour ablation: Devices, clinical applications and future directions. Int J Hyperthermia 2005;21:755–60.
- Ryan TP, Turner PF, Hamilton B. Interstitial microwave transition from hyperthermia to ablation: Historical perspectives and current trends in thermal therapy. Int J Hyperthermia 2010;26:415–33.
- Liang P, Yu J, Lu MD, Dong BW, Yu XL, Zhou XD, et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J Gastroenterol 2013;19:5430–8.
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia. 2010;26:305–15.
- Saccomandi P, Schena E, Silvestri S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int J Hyperthermia 2013;29:609–19.
- Di Costanzo GG, Francica G, Pacella CM. Laser ablation for small hepatocellular carcinoma: state of the art and future perspectives. World J Hepatol 2014;6:704–15.
- Orlacchio A, Bazzocchi G, Pastorelli D, Bolacchi F, Angelico M, Almerighi C, et al. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: Initial experience”. Cardiovasc Intervent Radiol 2008;31:587–94.
- Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia 2014;31:302–9.
- Stauffer PR, Goldberg SN. Introduction: Thermal ablation therapy. Int J Hyperthermia 2004;20:671–7.
- Kitchin D1, Lubner M, Ziemlewicz T, Hinshaw JL, Alexander M, Brace CL, et al. Microwave ablation of malignant hepatic tumours: Intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia 2014;30:299–305.
- Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: Long-term results in 117 patients. Radiology 2001;221:159–66.
- Liu F, Liang P, Yu X, Lu T, Cheng Z, Lei C, et al. A three-dimensional visualisation preoperative treatment planning system in microwave ablation for liver cancer: A preliminary clinical application. Int J Hyperthermia 2013;29:671–7.
- Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011;98:1210–24.
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encoutered in a multicenter study. Radiology 2003;226:441–51.
- Livraghi T. Radiofrequency ablation of hepatocellular carcinoma. Surg Oncol Clin N Am 2011; 20:281–99.
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Thermal ablation therapy for hepatocellular carcinoma: Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy. Hepatogastroenterology 2006;53:651–4.
- Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: Long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 2014;270:900–9.
- Zhang F, Wu G, Sun H, Ding J, Xia F, Li X, et al. Radiofrequency ablation of hepatocellular carcinoma in elderly patients fitting the Milan criteria: A single centre with 13 experience. Int J Hyperthermia 2014;30:471–9.
- Chopra S, Dodd GD III, Chanin MP, Chintapalli KN. Radiofrequency ablation of hepatic tumors adjacent to the gallbladder: Feasibility and safety. Am J Roentgenol 2003;180:697–701.
- Choi D, Lim HK, Kim MJ, Kim SH, Lee WJ, Kim SH, et al. Therapeutic efficacy and safety of percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the gastrointestinal tract. Am J Roentgenol 2004;183:1417–24.
- Zhang D, Xie D, Wei X, Zhang D, Chen M, Yu X, et al. Microwave ablation of the liver abutting the stomach: Insulating effect of a chitosan-based thermosensitive hydrogel. Int J Hyperthermia 2014;30:126–33.
- Zhang M, Liang P, Cheng ZG, Yu XL, Han ZY, Yu J. Efficacy and safety of artificial ascites in assisting percutaneous microwave ablation of hepatic tumours adjacent to the gastrointestinal tract. Int J Hyperthermia 2014;30:134–41.
- Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, et al. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J Gastroenterol 2015;21:1554–66.
- Hasegawa T, Takaki H, Miyagi H, Nakatsuka A, Uraki J, Yamanaka T, et al. Hyaluronic acid gel injection to prevent thermal injury of adjacent gastrointestinal tract during percutaneous liver radiofrequency ablation. Cardiovasc Intervent Radiol 2013;36:1144–6.
- Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: Safety and technical efficacy in 143 patients. Eur Radiol 2009;19:2630–40.
- Yamakado K, Nakatsuka A, Akeboshi M, Takeda K. Percutaneous radiofrequency ablation of liver neoplasms adjacent to the gastrointestinal tract after balloon catheter interposition. J Vasc Interv Radiol 2003;14:1183–6.
- Shao QJ, Han ZY, Ni XX, Sun WY, Sun YY, Hong L, et al. Feasible temperature of percutaneous microwave ablation of dog liver abutting the bowel. Int J Hyperthermia 2011;27:124–31.
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: Five-year experience. Ann Surg 2009;250:187–96.
- Wang KF, Pan W, Wang F, Wang GF, Madhava P, Pan HM, et al. Geometric optimization of a mathematical model of radiofrequency ablation in hepatic carcinoma. Asian Pac J Cancer Prev 2013;14:6151–8.
- Park MJ, Kim YS, Rhim H, Lim HK, Lee MW, Choi D. A comparison of US-guided percutaneous radiofrequency ablation of medium-sized hepatocellular carcinoma with a cluster electrode or a single electrode with a multiple overlapping ablation technique. J Vasc Interv Radiol 2011;22:771–9.
- Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma an analysis of 1000 cases. Cancer 2005;103:1201–9.
- Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: Correlation between local tumor progression after ablation and ablative margin. Am J Roentgenol 2007;188:480–8.
- Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Sakamoto A, Henmi S, et al. Percutaneous radiofrequency ablation therapy for hepatocellular carcinoma: A proposed new grading system for the ablative margin and prediction of local tumor progression and its validation. J Gastroenterol 2011;46:1418–26.
- Zervas NT, Kuwayama A. Pathological characteristics of experimental thermal lesions. Comparison of induction heating and radiofrequency electrocoagulation. J Neurosurg 1972;37:418–22.
- Goldberg SN, Kruskal JB, Oliver BS, Clouse ME, Gazelle GS. Percutaneous tumor ablation: Increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumor model. Radiology 2000;217:827–31.
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007;72:S124–S31.
- Vallone P, Catalano O, Izzo F, Siani A. Combined ethanol therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc Intevent Radiol 2006;29:544–51.
