Case study
Almost all clinical applications of hyperthermia have utilised a ‘local’ heating protocol whereby the heating (usually through the use of microwaves or ultrasound) is directed to tumour tissue, excluding as much normal tissue as much as possible. However, even with such ‘local’ techniques, through blood flow (BF) and some conduction, heat travels away from the tumour, making it difficult to maintain the desired temperature. Here we report on the first patient enrolled in a prospective clinical trial entitled ‘Body Warming to Alter (thermo) Regulation and the Microenvironment (B-WARM) therapy: a pilot study’ where heating is applied to the surface area of the thorax (regardless of tumour location which is usually away from the thorax) using water-filtered IR-A radiation with the intention of mildly increasing core temperature (Clinicaltrials.gov ID: NCT01896778). Based on our previous murine data, we hypothesised that the body’s natural thermoregulatory response to an increase in core temperature would result in a strong vascular response designed to remove heat from the core and dissipate it at the surface of the body [Citation1–Citation3]. In murine models we found that placing tumour-bearing mice in a warmer environment resulted in an increase in body temperature of several degrees, and was associated with a prolonged improvement of tumour vascular perfusion (even after body temperature returned to normal) [Citation1]. We also observed reduced hypoxia and interstitial fluid pressure ultimately leading to improved efficacy of chemotherapy and radiation of the tumour [Citation2,Citation3].
The patient was an otherwise healthy 66-year-old white male with a very large (14 cm) squamous cell carcinoma of the head and neck (SCCHN), involving the left neck from an unknown primary location (TXN3M0). The patient noted the mass approximately 6 months prior to presentation but allowed it to grow and did not seek medical attention. Pathology from fine needle aspiration did not allow formal human papilloma virus testing, though P16 positive cells were found. Informed written consent was obtained prior to participation and the study was approved by our institutional review board.
The thoracic region of the patient was exposed to skin-compatible infrared heat using the Heckel 3000 water-filtered IR-A radiation system (Esslingen, Germany) for 2 h, which was seen to increase core body temperature to approximately 39 °C. Importantly, the tumour itself was not the focus of the direct application of infrared treatment, but was expected to be exposed indirectly to an increase in temperature through BF, as was the rest of the body. The patient tolerated the procedure extremely well. He slept comfortably for most of the procedure. The patient received intravenous saline, and oral fluids were available on request but were needed rarely. There was a minimal feeling of ‘light headedness’ reported upon standing at the end of the procedure that resolved within seconds and was not accompanied by any observed unsteadiness. In general, though profusely covered in sweat, the patient reported feeling quite well and relaxed after the procedure.
Perfusion computed tomography (CT) was performed to assess changes in tumour BF, blood volume (BV) and permeability surface area product (PS) following mild systemic heating. Scans were performed 2 days prior to heating, immediately, and 5 days post heating. All perfusion CT was performed using a 16-row multi-slice CT scanner (General Electric, Light Speed RT16) software version qin.3, in the Department of Radiation Medicine at Roswell Park Cancer Institute. For perfusion imaging, 50 mL of non-ionic iodinated contrast agent (320 mg/mL, Visipaque, Amersham Health, Princeton, NJ) was injected at a rate of 4 mL/s. Images were acquired with the following parameters: Scan option cine mode, rotation direction clockwise, matrix size 512 × 512, slice number 16, slice thickness 2.5 mm, X-ray tube voltage 80 kV, X-ray tube current 100 mA. Perfusion data was processed using a commercial software package (Perfusion 3; GE Medical Systems) based on the accurate deconvolution algorithm [Citation4]. Generation of functional maps and measurements for BF, BV, and PS was performed using the central volume principle [Citation4]. Measurements were performed for five central slices of the tumour. Reported box and whisker graphs represent the 25th to 75th percentiles (box), median value (line), in addition to the minimum and maximum (whiskers) of the five slices.
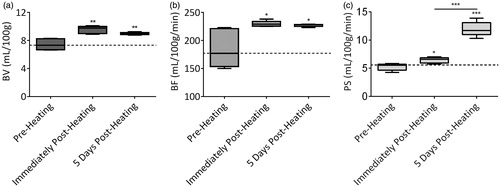
Pre-heating parametric BV maps showed variable levels of blood within the tumour (, left). A dramatic increase in tumour BV was observed immediately following heating (, middle). This increase was sustained even at 5 days following heating, although a slight reduction in BV was observed for some regions of the tumour (, right). A similar trend was also observed for parametric BF () and PS () maps. Quantification of BV levels revealed a ∼28% (p = 0.0011) increase immediately following heating compared to pre-heating levels, with a ∼21% (p = 0.0028) increase in BV levels maintained at 5 days following heating (). An increase in tumour BF was also observed immediately (∼24%, p = 0.0207) and 5 days (∼23%, p = 0.0259) post-heating (). Interestingly, an increase in tumour PS was observed both immediately (∼23%, p = 0.0121) and at 5 days (∼128%, p < 0.0001) post-heating ().
Figure 1. Temporal changes in vascular function following an increase in core body temperature in a patient with SCCHN (outlined in white). The panel of images represent parametric (a) blood volume (BV), (b) blood flow (BF) and (c) permeability surface (PS) maps calculated from perfusion CT scans at pre-heating (left), immediately post-heating (middle) and 5 days post-heating (right). Colour scale illustrates the increase in tumour BV, BF, and PS post-heat treatment.
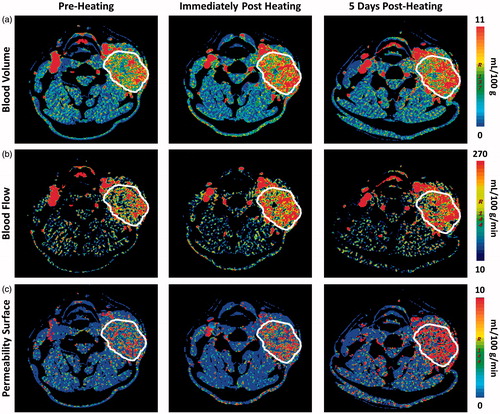
Figure 2. Perfusion parameters at pre- and post-heating calculated from perfusion CT in the patient with SCCHN. A region of interest was manually drawn along the margins of the tumour for calculation of (a) blood volume (mL/100 g), (b) blood flow (mL/100 g/min) and (c) permeability surface (mL/100 g/min). *p < 0.05, **p < 0.01, ***p < 0.0001 (Mann–Whitney test).
Following the 5-day post-heating scan, the patient was started on concurrent chemoradiation therapy consisting of cisplatinum 100 mg/m2 given every 3 weeks for 2 cycles and unilateral left neck radiation to 70 Gy in 35 fractions using opposed anterior/posterior fields. The supraclavicular fossa was concurrently treated with 50 Gy in 25 fractions using a matched low neck field. Target delineation and follow-up were as previously described [Citation5].
In our clinical practice, patients with such large masses as this usually achieve a complete clinical response only after a full 7-week course of chemoradiation therapy and the full response usually takes 19 weeks from the beginning of therapy. In this case, the tumour was noted to be reduced 30% after only two doses of radiation therapy and prior to his first dose of chemotherapy. There was an additional 40% reduction in the tumour mass noted with the next visit after a total of seven radiation treatments and a single chemotherapy infusion. Thereafter the patient continued to demonstrate gradual decrease in the size of the tumour mass. He is currently without evidence of disease and doing well in follow-up at 2 years.
Discussion
Thermoregulation in normal tissues is a rapidly reversible process that is mediated by the presence of a structurally mature vascular network (covered by smooth muscle cells) with autonomic innervation that effectively controls heat distribution [Citation6]. Members of our team have long been interested in utilising the impact of thermoregulatory responses in normal vascular beds to influence properties such as tumour BF in addition to various immune responses which are stimulated by mild elevations in body temperature [Citation1,Citation3,Citation7–11].
Elevated tumour interstitial fluid pressure (IFP) [Citation12] and hypoxia [Citation13] are both known to be poor prognostic factors in a variety of tumour types [Citation14,Citation15]; however, drugs and other therapies are not consistently able to overcome these problems. Murine data from our group shows that increasing core body temperature to approximately 39 °C results in a strong thermoregulatory response that increases BF throughout the body, including to the tumour, and is associated with reduced hypoxia and interstitial fluid pressure [Citation3,Citation10]. These data demonstrate that it may not be necessary to heat just the tumour in order to achieve clinically beneficial effects in the tumour microenivonment. Recently we have also demonstrated reduced hypoxia and IFP in a murine head and neck cancer model [Citation2]. Consistent with these preclinical observations, using perfusion CT, we demonstrate for the first time the ability of simply increasing core temperature (resulting in this case, from an application field of infrared heat that did not even include the tumour), to modulate tumour vascular function in patients.
Perfusion CT has previously been used to measure BF as a surrogate for tumour oxygenation [Citation16]. Perfusion CT parameters have also been shown to be predictive of response to chemotherapy and radiation in a variety of tumour types [Citation17]. In these studies generally, improved BF to the tumour was correlated with improved response to chemotherapy and/or radiotherapy. Our study shows that a modest increase in core temperature leads to an increase in tumour BV, BF and PS. Changes in these perfusion parameters were maintained even at 5 days, suggesting a sustained response to this treatment. Together, these findings suggest that vascular responses in the body to an increase in core temperature increase the delivery of blood to the tumour by increasing vessel diameter and BF.
Conclusion
In conclusion, it is feasible to utilise mild elevations in core body temperature to 39 °C, achieved through applications of mild heat to the surface of the body in an application field which did not include the head or neck, to produce durable changes in tumour perfusion. The impact of this temperature-induced vascular modulation on the efficacy of radiotherapy or chemotherapy requires further evaluation. We plan to test this effect in different tumour types with two durations of heating (30 and 120 min) In those tumour types with increased BF after mild systemic heating we plan to test the optimal timing and safety of combining heating with chemotherapy and/or radiation therapy. Immune analyses will also be performed. Once established, we feel that this process can be repeated even in small- to medium-sized clinics as minimal staff could be utilised to monitor multiple patients. Unlike other hyperthermia techniques, only simple core temperature readings are required with this method, obviating the need for complex temperature measurement. A single instructional session with an expert at using the device was all that was needed prior to this unobserved patient session.
Acknowledgements
This work was supported by a grant from the Dr med hc Erwin Braun Foundation and the Roswell Park Alliance Foundation. This work also utilized core resources supported by the NCI Cancer Center Support Grant P30-CA016056 (Johnson, CS).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Xu Y, Choi J, Hylander B, Sen A, Evans SS, Kraybill WG, et al. Fever-range whole body hyperthermia increases the number of perfused tumor blood vessels and therapeutic efficacy of liposomally encapsulated doxorubicin. Int J Hyperthermia 2007;23:513–27.
- Winslow TB, Eranki A, Ullas S, Singh AK, Repasky EA, Sen A. A pilot study of the effects of mild systemic heating on human head and neck tumour xenografts: Analysis of tumour perfusion, interstitial fluid pressure, hypoxia and efficacy of radiation therapy. Int J Hyperthermia 2015;31:693–701.
- Sen A, Capitano ML, Spernyak JA, Schueckler JT, Thomas S, Singh AK, et al. Mild elevation of body temperature reduces tumor interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumor models. Cancer Res 2011;71:3872–80.
- Bisdas S, Baghi M, Wagenblast J, Knecht R, Thng CH, Koh TS, et al. Differentiation of benign and malignant parotid tumors using deconvolution-based perfusion CT imaging: feasibility of the method and initial results. Eur J Radiol 2007;64:258–65.
- McCloskey SA, Jaggernauth W, Rigual NR, Hicks WL Jr, Popat SR, Sullivan M, et al. Radiation treatment interruptions greater than one week and low hemoglobin levels (12 g/dL) are predictors of local regional failure after definitive concurrent chemotherapy and intensity-modulated radiation therapy for squamous cell carcinoma of the head and neck. Am J Clin Oncol 2009;32:587–91.
- Guyton A, Hall J. Textbook of medical physiology, 11th ed. Philadelphia, PA: Elsevier, 2006, pp. 899–900.
- Burd R, Dziedzic TS, Xu Y, Caligiuri MA, Subjeck JR, Repasky EA. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol 1998;177:137–47.
- Wang XY, Ostberg JR, Repasky EA. Effect of fever-like whole-body hyperthermia on lymphocyte spectrin distribution, protein kinase C activity, and uropod formation. J Immunol 1999;162:3378–87.
- Dayanc BE, Beachy SH, Ostberg JR, Repasky EA. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia 2008;24:41–56.
- Lee CT, Mace T, Repasky EA. Hypoxia-driven immunosuppression: a new reason to use thermal therapy in the treatment of cancer? Int J Hyperthermia 2010;26:232–46.
- Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res 2013;1:210–16.
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer 2004;4:806–13.
- Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis 2009;26:19–34.
- Taghian AG, Abi-Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol 2005;23:1951–61.
- Fyles A, Milosevic M, Pintilie M, Syed A, Levin W, Manchul L, et al. Long-term performance of interstial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiother Oncol 2006;80:132–7.
- Haider MA, Milosevic M, Fyles A, Sitartchouk I, Yeung I, Henderson E, et al. Assessment of the tumor microenvironment in cervix cancer using dynamic contrast enhanced CT, interstitial fluid pressure and oxygen measurements. Int J Radiat Oncol Biol Phys 2005;62:1100–7.
- Petralia G, Bonello L, Viotti S, Preda L, d’Andrea G, Bellomi M. CT perfusion in oncology: how to do it. Cancer Imaging 2010;10:8–19.
