Abstract
Objective. To explore the negative predictive value (NPV), positive predictive value (PPV), sensitivity, and specificity of natriuretic peptides, cut-off levels, and the impact of gender and age in elderly patients with systolic heart failure (HF). Design. Cross-sectional exploratory study. Setting. One primary healthcare centre. Patients. A total of 109 patients with symptoms of HF were referred for echocardiographic examination with a cardiovascular consultation. Systolic HF was diagnosed (ESC guidelines) in 48 patients (46% men, 54% women, mean age 79 years) while 61 patients (21% men, 79% women, mean age 76 years) had no HF. Main outcome measures. NPV, PPV, sensitivity, specificity, and cut-off levels. Results. Including all 109 patients, NPV was 88% for NT-proBNP (200 ng/L) and 87% for BNP (20 pg/ml). PPV was 81% for NT-proBNP (500 ng/L) and 68% for BNP (50 pg/ml). Sensitivity was 96% for NT-proBNP (100 ng/L) and 96% for BNP (10-20 pg/ml). Specificity was 87% for NT-proBNP (500 ng/L) and 71% for BNP (50 pg/ml). Nt-proBNP (β = 0.035; p < 0.001) and BNP (β = 0.030; p < 0.001) were associated with age, but not with gender. In a multivariate analysis age (β = 0.036; p < 0.001) and male gender (β = 0.270; p = 0.014) were associated with NT-proBNP, but only age for BNP (β = 0.030; p < 0.001). Conclusion. Natriuretic peptides in an elderly population showed high NPVs, but not as high as in younger patients with HF in other studies. Age and male gender were associated with higher levels of NT-proBNP while only age was related to elevated BNP levels.
Knowledge of the utility of natriuretic peptides in elderly patients with suspected heart failure in primary healthcare is still limited.
Natriuretic peptides showed high negative predictive values, but not as high as in younger patients with heart failure in other studies.
Natriuretic peptides are useful as diagnostics tools even in elderly patients but the most precise cut-off levels still have to be determined.
Age and male gender were associated with higher levels of NT-proBNP, while only age was related to elevated BNP levels.
Natriuretic peptides (NT-proBNP and BNP) have emerged as valuable tools in excluding HF [Citation1,Citation2], mostly in patients with new onset and untreated heart failure (HF). Accordingly, the European Society of Cardiology (ESC) has recommended the use of natriuretic peptides in a diagnostic algorithm in their 2005 guidelines, mainly to rule out HF [Citation3]. These recommendations are based on studies with patients 70 years of age and younger [Citation1,Citation4]. They underline the importance of recognizing female gender and increasing age when setting cut-off points [Citation5]. Elderly, and especially elderly female patients with HF, are more often treated by general practitioners than by cardiologists [Citation6,Citation7]. In primary healthcare there is a strong need for easily accessible and accurate diagnostic methods, as availability of echocardiography may be limited.
The present study was performed because there are still unresolved questions regarding the diagnostic accuracy and cut-off values of natriuretic peptides in elderly patients in primary healthcare (PHC) with suspected HF [Citation8].
The aim of this study was, primarily, to explore the sensitivity, specificity, negative and positive predictive values (NPV, PPV) of NT-proBNP and BNP in a cross-sectional study of primary health care patients with systolic HF and, secondarily, to evaluate the impact of age and gender on natriuretic peptides levels.
Material and methods
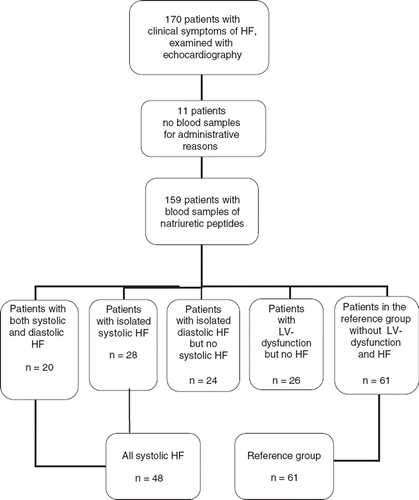
Patients were recruited from one selected PHC in northern Sweden between 2000 and 2003. The PHC has a catchment area of approximately 7800 inhabitants of whom many are of advanced age. These demographics are probably representative of those in ordinary Swedish cities. For many years a computer-based registry of all patients with different diagnoses has been used by the PHC. In 2001, this registry included on clinical grounds 150 patients with a suspected diagnosis of HF. The present study comprised registry patients consecutively identified by the GP at the PHC, as well as incident patients with suspected HF during the recruitment period. Patients with dementia, terminal illness, or those who declined participation were excluded. Originally there were 170 patients whose characteristics have been described earlier [Citation9]. For a flowchart of the patient population, see .
All participants had symptoms (mainly dyspnoea) indicating chronic HF and were evaluated clinically by a GP before being referred for echocardiography (MO) and subsequent cardiovascular consultation. The study cardiologist (KB) confirmed or refuted the diagnosis of HF based on the GP's pre-specified HF record, echocardiography results, and hospital records. For the present study we included patients diagnosed with systolic HF (48 patients) with or without diastolic HF, while the reference group (61 patients) consisted of patients with no HF (see ).
Left ventricular systolic and diastolic function
The diagnosis of systolic HF was established according to the ESC guidelines [Citation10]. Global left ventricular systolic function was assessed as normal, mildly, moderately, or severely depressed. Normal systolic function corresponded to an ejection fraction (EF) of ≥ 55% and severe systolic function was considered to be an EF < 30%. Left ventricular diastolic function was assessed according to definitions proposed by Bergstrom et al. [Citation11]. If any of the diastolic variables were abnormal then diastolic dysfunction was established. Patients diagnosed with LV dysfunction had abnormal systolic and/or diastolic LV function without symptoms dependent of HF.
Blood sampling and laboratory measurements
Blood samples (plastic EDTA tubes) for analysis of natriuretic peptides were taken in fasting patients who had rested for 20 minutes. After five minutes the samples were centrifuged for 10 minutes at 4° C and then stored frozen at −70° C. NT-proBNP was analysed with Roche Elecsys proBNP immunoassay [Citation12]. BNP was analysed with an immunoradiometric assay (Shionoria) with a coefficient of variation (CV) from 2.0 within run and 4.2 between run. The Food and Drug Administration (FDA) recommended cut-off values of 125 pg/ml for patients younger than 75 years and 450 pg/ml for patients 75 years and older to diagnose individuals suspected of having congestive HF for the Roche Elecsys NT-proBNP immunoassay [Citation13].
We explored cut-off values for NT-proBNP from 100 to 500 ng/L which were partly based on FDA recommendations [Citation13]. To establish corresponding BNP cut-off values we analysed the quotient between NT-proBNP and BNP in patients with both systolic and diastolic HF who had the highest median levels. We derived a quotient of approximately 10 between NT-proBNP (mean level 1258 ng/L) and BNP (mean level 119 pg/ml), which resulted in cut-off values for BNP from 10 to 50 pg/ml.
Statistics
Mean values and standard deviations (SD) for baseline characteristics were calculated for continuous variables while natriuretic peptides were expressed as median, minimum, and maximum values and also log transformed as they were not normally distributed. The non-parametric test, the Mann–Whitney U-test, was used to test levels of NT-proBNP and BNP in the different categories of HF and LV dysfunction compared with the reference group. NPV, PPV, sensitivity, and specificity were calculated. Odds ratio (OR) with 95% confidence interval (CI) was determined for having HF at different cut-off values. For clinical reasons we chose age and gender as covariates in a linear regression analysis. The null hypothesis was rejected if the p-value was less than 0.05. Statistical analyses were carried out using SPSS version 15.0.
Results
Baseline characteristics for 48 patients with systolic HF and 61 patients in the reference group are presented in . Results of NPV, PPV, sensitivity, and specificity for different cut-off levels of N T-proBNP and BNP in these 109 patients are presented in and the impact of gender and median age are presented in and . With a prevalence of 44% the highest value of NPV and sensitivity was found at a cut-off level of 200 ng/l and 20 pg/ml, for NT-proBNP and BNP, respectively, not taking gender or age into account. For NT-proBNP, PPV was 57% (CI 51-63). NPV 88% (CI 73-95), sensitivity 92% (CI 81-97) and specificity 46% (CI 34-58) at 200 ng/l. At 20 pg/ml for BNP corresponding values and CI were PPV 49% (45-53), NPV 87% (61-97), sensitivity 96% (87-99) and specificity 21% (12-33), respectively.
Table I. Baseline characteristics, comorbidities, and treatment of 48 patients with systolic HF and 61 patients in the reference group.
Table II. Negative predictive value (NPV), positive predictive value (PPV), sensitivity, and specificity, respectively, and odds ratio (OR) and its confidence interval (CI) of having HF at different cut-off values for NT-proBNP and BNP in 48 patients with systolic HF and 61 referent patients.
Table III. Negative predictive value (NPV), positive predictive value (PPV), sensitivity (sens) and specificity (spec), of different cut-off values for NT-proBNP.
Table IV. Negative predictive value (NPV), positive predictive value (PPV), sensitivity (sens) and specificity (spec), of different cut-off values for BNP.
For all 109 patients a linear regression analysis showed that age was significantly associated with higher levels of both NT-proBNP (β = 0.035; p < 0.001) and BNP (β = 0.030; p < 0.001), while gender was not (β = 0.219; p = 0.076 and β = 0,111; p = 0.275 respectively). In a multiple linear regression analysis both age (β = 0.036; p < 0.001) and male gender (β = 0.270; p = 0.014) were significantly associated with higher levels of NT-proBNP, while only age was significantly associated with increasing levels of BNP (β = 0.030; p < 0.001).
Discussion
The main finding in this study was that the highest value of sensitivity and NPV for both NT-proBNP and BNP was reached at a cut-off level of 200 ng/l and 20 pg/ml respectively, not taking gender or age into account. In this study we chose to evaluate only patients with systolic HF because evidence-based treatment has firmly been established in this category of patients [Citation3]. Many controversies regarding how to diagnose and treat diastolic HF remain. For a general practitioner NPV is considered to be the most clinically relevant value, that is, to exclude patients with systolic HF.
The strength of this study is that all patients were diagnosed with echocardiography according to ESC guidelines and that their treatment was specified. This was a small and exploratory study with patients from one PHC with rather high prevalence (44%), which limits general conclusions. This is not an epidemiological study, rather a clinical study of elderly patients who are representative of general clinical practice.
Fuat et al. [Citation14] compared the utility of BNP (Biosite Triage System) and NT-proBNP (Roche Diagnostics) in diagnosing systolic HF in patients referred from 94 different GPs. In this study 114 patients (38%) had left ventricular systolic dysfunction and the best NPV (92%, CI 86–98) for NT-proBNP was found to be 150 pg/ml and 40 pg/ ml for BNP (88%, CI 80–96), with a specificity of 40% and 38%, respectively. Their results for NPV were comparable with ours. Their patients were, however, younger, more often women and more patients had systolic HF. Also, a different BNP method was used.
Gustafsson et al. [Citation4] evaluated NT-proBNP using the FDA's recommendations. Their highest value for both NPV and sensitivity was 100% at a cut-off level of 125 pg/ml, while our highest values of NPV and sensitivity were 88% and 92%, respectively, using a cut-off level 200 ng/L. Possible explanations for these differences could be that our patients were almost 10 years older, or differences in medical therapy.
Zaphiriou and coworkers [Citation15] compared N T-proBNP (Roche) and BNP (Biosite) using a cut-off for NT-proBNP of 125 pg/ml and 100 pg/ml for BNP. For NT-proBNP they reported a NPV of 97%, sensitivity 98%, and specificity 35%, while for BNP the corresponding figures were 87%, 79%, and 72%, respectively, showing that a lower cut-off for BNP would provide better diagnostic utility. Our best cut-off value for BNP (Shionoria) was 20 pg/ml, also suggesting that 100 pg/ml is too high, but the methods for BNP differ in these studies which is very important to consider when comparing study results. Compared with other studies we found somewhat lower values for sensitivity and NPV. Possible explanations may be that our patients were older and more extensively treated.
Hess et al. found that healthy females had higher levels of NT-proBNP than males and that advanced age increased levels of NT-proBNP in both genders. However, in patients with cardiac disorders they found that levels of NT-proBNP increased with higher age, but females did not demonstrate higher levels of NT-proBNP than males [Citation16]. Their patients were not diagnosed with echocardiography and the authors did not present data on medical treatment. In our study, conversely, the diagnosis of systolic HF was confirmed with echocardiography and treatment was defined.
Wang et al. [Citation17] found that both age and female gender were associated with increased levels of BNP. We found an association only for age but again our patients had systolic HF, were much older, and were more extensively treated.
If we apply the present ESC guidelines [Citation18] to rule out HF (NT-proBNP < 400 pg/ml), 12/48 (25%) of patients would be classified as false negative. Gender stratification reveals that 9/26 (35%) women and 3/22 (14%) of the men would be classified as false negative.
Clinical implications
Diagnostic studies on the usefulness of natriuretic peptides in elderly patients at PHCs are relatively sparse. The utility of these new diagnostic tools may be more limited compared with younger patients with suspected HF due to the influence of age, frequent comorbidities, and polypharmacy. Despite the limitations of the present study our findings demonstrate high NPV values, albeit not as high as reported in other studies with younger patients and those presenting in emergency departments. Patients in a PHC setting, as in our study, are often elderly and treated for multiple diseases. ESC guidelines, on the other hand, refer to untreated patients. For this reason a cut-off level of NT-proBNP of 200 pg/ml seems more appropriate instead of the recommended value of 400 pg/ml.
Of significant importance is the fact that the reference group was not a control group of healthy people. All patients in this study had symptoms suggestive of HF, but the reference group was diagnosed not to have HF. This is a common clinical scenario in a GP's daily practice, while we feel that the reference group should not consist of healthy people. However, future studies with larger patient cohorts are needed to sort out confounding factors in order to establish optimal cut-off values for NT-proBNP and BNP in elderly PHC patients.
Conclusions
Our analysis indicated that values of 200 ng/L for NT-proBNP and 20 pg/ml for BNP were the preferred cut-off values to rule out HF in elderly PHC patients with clinical symptoms of HF. Natriuretic peptides in an elderly population showed high NPVs although not as high as found in younger populations.
Increasing age and male gender were associated with higher levels of NT-proBNP while only increasing age was related to elevated BNP levels.
As in all other patient groups, natriuretic peptides should always be evaluated from a perspective that considers clinical symptoms and pre-test probability. This will be even more important in elderly patients with multiple factors and high prevalence of the disease that impact on the predictive values of natriuretic peptides.
Acknowledgements
This study was supported by a research grant from Västerbotten County Council, Foundation of Medical Research, Skellefteå and an unrestricted grant by Novartis Sweden AB. The authors would like to thank MD Thomas Suh, Skellefteå, for linguistic assistance.
Patients signed written consent for the study and the study was approved by the Committee of Ethics at Umeå University.
The authors have no conflict of interest.
References
- Cowie MR, Jourdain P, Maisel A, Dahlstrom U, Follath F, Isnard R, . Clinical applications of B-type natriuretic peptide (BNP) testing. Eur Heart J 2003;24:1710–18.
- Bettencourt PM. Clinical usefulness of B-type natriuretic peptide measurement: present and future perspectives. Heart 2005;91:1489–94.
- Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, . Guidelines for the diagnosis and treatment of chronic heart failure: Executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26:1115–40.
- Gustafsson F, Steensgaard-Hansen F, Badskjaer J, Poulsen AH, Corell P, Hildebrandt P. Diagnostic and prognostic performance of N-terminal ProBNP in primary care patients with suspected heart failure. J Card Fail 2005;11:S15–20.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC, Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002;40:976–82.
- Fagerberg B. [National and international guidelines ignore significant cause of heart failure. Diastolic dysfunction is often the cause of heart failure in the elderly and in women]. Lakartidningen 1998;95:5305–8.
- Rutten FH, Grobbee DE, Hoes AW. Diagnosis and management of heart failure: A questionnaire among general practitioners and cardiologists. Eur J Heart Fail 2003;5:345–8.
- Schaufelberger M, Bergh CH, Caidahl K, Eggertsen R, Furenas E, Lindstedt G, . Can brain natriuretic peptide (BNP) be used as a screening tool in general practice? Scand J Prim Health Care 2004;22:187–90.
- Olofsson M, Edebro D, Boman K. Are elderly patients with suspected HF misdiagnosed? A primary health care center study. Cardiology 2006;107:226–32.
- Cardiology TESo. Guidelines for the diagnosis of heart failure. Eur Heart J 1995;16:741–51.
- Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC). Eur J Heart Fail 2004;6:453–61.
- Roche. Roche Diagnostic Corporations 11/12/02 51 O(k) Summary K022516-Elecsys ProBNP NOV 1 9 2002 Introduction. 2002.
- FDA. Available at: http://www.fda.gov/cdrh/pdf2/k022516. (18 December 2002).
- Fuat A, Murphy JJ, Hungin AP, Curry J, Mehrzad AA, Hetherington A, . The diagnostic accuracy and utility of a B-type natriuretic peptide test in a community population of patients with suspected heart failure. Br J Gen Pract 2006;56:327–33.
- Zaphiriou A, Robb S, Murray-Thomas T, Mendez G, Fox K, McDonagh T, . The diagnostic accuracy of plasma BNP and NT-proBNP in patients referred from primary care with suspected heart failure: Results of the UK natriuretic peptide study. Eur J Heart Fail 2005;7:537–41.
- Hess G. N-Terminal pro-brain Natriuretic Peptide (NT-proBNP) in healthy blood donors and in patients from general practitioners with and without a diagnosis of cardiac disease. Clin Lab 2005;51:167–72.
- Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PW, . Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002;90:254–8.
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–442.