Abstract
Objective. To study one-year incidence and risk factors of severe hypoglycaemias (SH) in adult drug-treated diabetic patients living in two Finnish communities. Design. The episodes of SH and their risk factors were identified from local ambulance registers, from the databases of local health care units, and from patient questionnaires. Setting. The target population consisted of all drug-treated diabetic patients from the two middle-sized communities in southern Finland, altogether 1776 patients. The study was retrospective. Subjects. A total of 1469 patients (82.7% of the target population) gave informed consent for the use of their medical records and 1325 patients (74.6% of the target population) returned the detailed 36-item questionnaire. Results. Of type 1 and type 2 insulin-treated diabetic patients, 14.6% and 1.0%, respectively, needed ambulance or emergency room care (incidence of 30.5 and 3.0 per 100 patient years). However, 31.0% of type 1 and 12.3% of type 2 diabetic patients reported at least one episode of SH (incidence of 72.0 and 27.0 per 100 patient years). Of all insulin-treated patients, 53 (7.8%) reported three or more episodes of SH. Significant independent risk factors for SH were depression, daily exercise, and nephropathy but not glycaemic control. Conclusion. The incidence of SH was high in both types of insulin-treated diabetic patients. However, the recurrent episodes of SH were clustered in a small minority of insulin-treated patients with diabetes. The risk of SH should be considered when assessing the treatment target for an individual diabetic patient.
The risk of severe hypoglycaemia (SH) has not been thought to be a problem in the treatment of type 2 diabetes.
In this population-based study cohort severe hypoglycaemias in both type 1 and type 2 insulin-treated diabetic patients seemed to be more common than previously thought.
Severe hypoglycaemias seem, however, to be clustered in a small minority of insulin-treated diabetic patients.
The risk of severe hypoglycaemia should be considered when assessing the treatment target for an individual diabetic patient.
The risk of severe hypoglycaemias (SH) or the fear of them poses the greatest obstacle to achieving good glucose control in insulin-treated diabetic patients [Citation1,Citation2]. The population-based studies of the epidemiology of SH in patients with both type 1 and type 2 diabetes are quite limited and somewhat controversial [Citation3–7]. Intensive antihyperglycaemic therapy and unawareness of hypoglycaemic symptoms increase the risk of SH in patients with type 1 diabetes [Citation8–11], although this has not been the case in all studies [Citation12,Citation13].
Recent prospective clinical trials with very intensive glucose lowering have also shown an increased risk of SH in patients with type 2 diabetes [Citation14,Citation15], although some clinical trials have suggested that the risk of SH is not a problem in insulin-treated patients with type 2 diabetes [Citation16–19] and may be related to the insulin regimen used [Citation7]. Use of sulphonylureas may increase the risk of SH, especially in old patients [Citation7].
In our retrospective population-based study we analysed the rate and risk factors of SH episodes, which were either self-reported or resulted in ambulance or emergency room care among diabetic residents of two Finnish communities during a one-year period.
Material and methods
Study patients
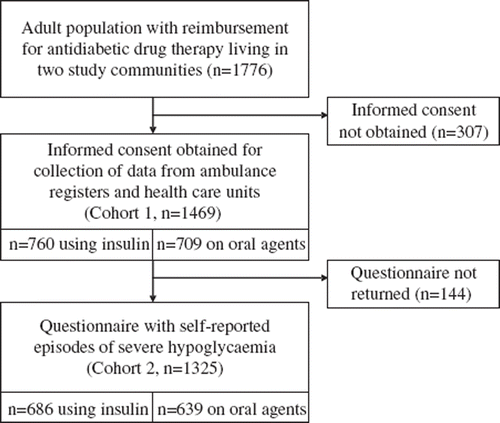
The study was carried out in two medium-sized communities. In the city of Kouvola (31 399 inhabitants; data from 2004), the primary diabetes care was based on family doctors. In Nurmijärvi (35 922 inhabitants; data from 2004), type 1 diabetic patients and most of the type 2 diabetic patients undergoing intensive treatment were treated by one primary care doctor. From the Reimbursement Register of the Social Insurance Institution of Finland we identified 1776 diabetic patients over 18 years of age and living either in Kouvola (951) or Nurmijärvi (827), who were eligible for reimbursement payments for antidiabetic medication. Of this target population (1776 subjects), 1469 (cohort 1: 82.7% of the eligible study population) provided their written consent for the use of their clinical data from different sources. Of these, 1325 study patients (cohort 2: 74.6% of the eligible population) also returned a questionnaire in which they reported the number of severe hypoglycaemias during the preceding 12 months (year 2005) and data on the risk factors for SH. The numbers of insulin-treated patients in cohorts 1 and 2 are shown in . At the time of the study, glargine insulin was practically the only long-acting insulin analogue available and reimbursed and was used by 56.6% of the study patients with type 1 diabetes. The demographic data of the study patients as well as their diabetes type are given in . Of type 1 diabetic patients 78.7% and almost all (97.7%) with type 2 diabetes were followed-up in primary health care.
Table I. Demographic data of the study patients (mean±SD).
Survey of severe hypoglycaemia episodes
SH was defined as a condition for which the patient needs the assistance of another person to recover from a hypoglycaemic episode as used by the UK Hypoglycaemia Study Group [Citation20]. In cohort 1 (1469 patients) data on the episodes of SH during 2005 were collected from the patient records used in the two primary health care centres and in the local hospitals with 24-hour emergency room service, and from the 24-hour ambulance service registers of these communities. In cohort 2 (1325 patients) the study patients were asked to report the number of SH episodes by answering the following question: “Have you needed help from another person to recover from an episode of low blood glucose concentration (hypoglycaemia or ‘insulin shock’) during the latest 12 months (year 2005)” using answer options “No or Yes, times”.
Measurement of HbA1c
The HbA1c value used in the data analysis was the mean of all HbA1c measurements of a patient during the year 2005. HbA1c was measured in local laboratories with immunological assays (Olympus analyzer® in Nurmijärvi and Roche Integra 800® analyzer in Kouvola). The correlation coefficient between the two assays was 0.96, but the assay used in Kouvola gave on average 0.6% units lower HbA1c values than the assay used in Nurmijärvi as reported earlier [Citation21].
Evaluation of risk factors for severe hypoglycaemia
In the 36-item questionnaire, the study patients reported data on weight, smoking status (current smoker or non-smoker), the weekly use of alcohol (the number of 15 g alcohol doses during a week), living status (alone or with other people), physical activity (sedentary, medium, or active), profession (agriculture/labour, white-collar, not in work), depression (no depression or self-reported feelings of depression or therapy for depression), the place of residence, the place of diabetes care (primary health care or hospital diabetes outpatient unit), the latest doctor's visit because of diabetes during the past 12 months (yes or no). Constant microalbuminuria (recurrent overnight albumin excretion > 20 μg/min) or more advanced renal disease (macroproteinuria or consistently elevated serum creatinine value) indicated nephropathy. The level of physical activity was grouped by the number of weekly episodes of exercise lasting at least half an hour and causing at least slight shortness of breath or sweating. The level of physical activity was considered “active” (exercise daily), “medium” (number of weekly exercise episodes from 2 to 6), or “sedentary” (exercise less than twice a week). The prevalence of depression was evaluated by asking the study patients: “Have you felt depressed during the latest year (2005) either (1) the whole time, (2) most part of time, (3) a notable part of the time, (4) sometimes, (5) only a little part of time, or (6) not at all?” Options 1-4 were regarded as showing a depressed mood. Current use of antidepressant medication was collected from patient records.
Statistics
All the data are given as mean ± standard deviation (SD). We used Student's t-test and a chi-squared test for between-group comparisons. The independent role of the risk factors for self-reported episodes of SH was analysed by using multivariate stepwise logistic regression analysis.
Ethics
The Ethics Committee of the Department of Internal Medicine in Helsinki Uusimaa Hospital District approved the study protocol.
Results
Severe hypoglycaemia requiring ambulance or emergency room care
From the ambulance registers and the patient records of local hospitals and health care centres we found altogether 100 episodes of SH in 47 patients (3.2% of all patients in cohort 1), of whom 46 were on insulin therapy (6.1% of all insulin-treated patients in cohort 1, ). Ambulance personnel treated 72 of 91 SH episodes on site, whereas 19 patients were subsequently transferred to emergency room care.
Table II. Number and incidence (per 100 patient years) of episodes of severe hypoglycaemia (SH) grouped by diabetes type and mode of treatment.
Self-reported episodes of severe hypoglycaemia
The total number of self-reported SH episodes in the study year was 340, of which 302 episodes occurred among 132 insulin-treated patients, and 38 episodes among 24 patients on oral therapy (see ). Altogether 31.0% of the patients with type 1 diabetes and 12.3% of those with type 2 diabetes reported at least one SH episode (the incidence rates are given in ). Of all study patients the recurrent episodes of SH were clustered among 56 (4.2%) patients, of whom 33, 20, and three had type 1, type 2, and secondary diabetes, respectively. There was no significant difference in the incidence of SH among patients living in the two communities studied.
Severe hypoglycaemia in relation to diabetes type and quality of basal insulin
The absolute number of self-reported SH episodes was almost equal to both major types of diabetes (see ) although 73% of SH episodes needing ambulance or emergency room service occurred in patients with type 1 diabetes. There was no statistically significant difference in the occurrence of SH between type 1 diabetic patients using either NPH (neutral protamine Hagedorn) or glargine insulin as basal insulin (data not shown).
Risk factors of severe hypoglycaemia
In the logistic multivariate analysis independent risk factors of SH were nephropathy, depression, active physical exercise, and follow-up of diabetic patients in secondary or tertiary care hospitals (). In contrast, HbA1c (OR [odds ratio] 1.063; 95% CI (confidence interval) 0.874–1.293), smoking or even alcohol use were not independent risk factors for SH. Other variables included in the multivariate analysis were the duration of the diabetes, the living status of the patient (alone or with other people), the place of residence (Kouvola vs. Nurmijärvi), diabetes controls by a doctor during the year (yes or no), diabetes type, and the mode of diabetes care (oral therapy, insulin, or their combination). It is worth noting that 11 (19%) diabetic patients with recurrent episodes (three or more) of SH were outliers of any diabetes care, since they had no diabetes-related visits to any doctor during the observation year [Citation22].
Table III. Risk factors for severe hypoglycaemia.
Discussion
In this population-based study, we have found that 31% of the patients with type 1 diabetes and 12% of the insulin-treated patients with type 2 diabetes had at least one episode of severe hypoglycaemia during a one-year period. The number of ambulance or emergency room treated SH episodes comprised just the tip of the iceberg, since only 6% of all insulin-treated patients needed intensive treatment for SH. Recurrent episodes of SH occurred in 8% of the insulin-treated patients. Of patients with type 2 diabetes using oral therapy only 4% reported a single episode of SH and only one patient needed ambulance care.
The strength of our study is the use of three different data sources to cover all possible episodes of SH in an unselected population. Moreover, data were collected from an observation period of one year. We found no difference in the overall incidence rate of SH between the communities although the primary health care of the insulin-treated patients was organized differently. Due to the retrospective nature of the study it is possible that patients under-reported or even over-reported the episodes of SH, and the understanding of the SH definition may have varied. Inaccuracy of recall of the rate of hypoglycaemia has previously been documented [Citation23], therefore the exact incidence of SH may still remain in the dark. Of all the SH episodes, the majority received treatment from another person without any action by health care personnel. Therefore, in everyday care, the majority of SH episodes may go undocumented in clinical databases. Of patients with recurrent SH, 19% were outliers of standard medical care (i.e. they had no visits to a doctor during the observation year because of their diabetes) although they seemed to have remarkable problems with their glycaemic control. Patients requiring ambulance care received treatment mostly at the hands of on-site ambulance personnel as has been shown previously [Citation24].
The overall incidence of SH among type 1 diabetic patients was much higher than observed in the Diabetes Control and Complications Trial (DCCT) and some other clinical trials [Citation4,Citation6]. This may be explained by the clustering of SH in patients who appeared to receive no structured diabetes care. Of note is that 69% of type 1 and 88% of insulin-treated type 2 diabetic patients did not experience a single SH episode. At the time of data collection, the use of long-acting insulin analogues started in Finland and was eligible for reimbursement only among type 1 diabetic patients. However, we observed no lower risk for SH among patients using long-acting insulin analogues than among those using NPH insulin as expected on the basis of clinical trials [Citation25]. At the time of the study all insulin-treated patients with type 2 diabetes still received NPH insulin.
The international guidelines for the treatment of diabetes have brought the goals of HbA1c levels closer to normoglycaemia in both type 1 and type 2 diabetes although recent data suggest that intensive therapy for type 2 diabetes may even increase mortality [Citation15]. Some have considered the occurrence of SH to be a minor problem in type 2 diabetes compared with type 1 diabetes [Citation16,Citation18], although observational studies have yielded contradictory results [Citation4,Citation6]. In line with these data, we found the rate of SH among insulin-treated type 2 diabetic patients to be about one-third to half of the rate of type 1 patients, but the absolute number of SH episodes was nearly equal within the two major types of diabetes.
Unawareness of hypoglycaemia, long diabetes duration, previous episodes of SH, strict glycaemic control, and male gender are well-known risk factors for SH [Citation7,Citation11,Citation26,Citation27]. We did, however, not find any negative correlation between the HbA1c level and the risk of SH, even if insulin-treated patients were analysed separately. This may be explained by the fact that SH episodes were clustered in some poorly controlled patients with high HbA1c. One weakness of our study was that we did not record data on awareness of hypoglycaemia in our patients.
Interestingly, depression and high daily physical activity turned out to be independent risk factors of SH. The connections between SH risk and depression as well as SH risk and abundant physical activity are findings not often reported in previous studies. Although the presence and severity of depression were evaluated only with one question in our 36-item questionnaire, we found an independent positive correlation between SH and depression. It still remains uncertain whether the depression is a partial cause or a consequence of the labile glycaemic control. A link between diabetes in general and depression has recently been found in other studies [Citation28–30] or common aetiological factors may exist behind these two disorders [Citation30]. Physically very active diabetic patients seemed to have a 2.5-fold increased risk of SH. Varying level of physical activity may be a challenge for tailoring personal insulin therapy [Citation31].
In conclusion, our population-based study revealed a high incidence of SH among both type 1 and insulin-treated type 2 diabetic patients. The recurrent episodes of SH were clustered in a minority of insulin-treated patients, many of them being outliers of any diabetes care. The risk of SH must always be considered among insulin-treated patients and the avoidance of hypoglycaemia should be a major target in their treatment.
Acknowledgements
This study benefited from the support of the Finnish Diabetes Research Foundation. The authors would like to thank the nurses at the Kouvola and Nurmijärvi Health Centres who participated in gathering the data.
Declaration of competing interests
Nothing to declare.
References
- Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–76.
- Davis S, Alonso MD. Hypoglycemia as a barrier to glycemic control. J Diabet Complications 2004;18:60–8.
- Henderson N, Allen KV, Deary IJ, Frier BM. Hypoglycaemia in insulin-treated type 2 diabetes: Frequency, symptoms and impaired awareness. Diabet Med 2003;20:1016–21.
- Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, . Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: A population-based study of health service resource use. Diabetes Care 2003;26:1176–80.
- Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, . Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: A population-based study. Diabet Med 2005;22:749–55.
- Frier BM. Hypoglycaemic valleys: An under-recognised problem in type 2 diabetes? Int J Clin Pract 2002;129:12–9.
- Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in type 2 diabetes. Diabet Med 2008;25:245–54.
- Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes 1997;46:271–86.
- Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER. Hypoglycemia in pregnant women with type I diabetes: Predictors and role of metabolic control. Diabetes Care 2008;31:9–14.
- Davis EA, Keating B, Byrne GC, Russell M, Jones T W. Impact of improved glycaemic control on rates of hypoglycaemia in insulin dependent diabetes mellitus. Arch Dis Child 1998;78: 111–5.
- Clarke W, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM: A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–22.
- Abrahamian H, Hornlein B, Gurdet C, Willinger C, Zaruba E, Irsigler K. Insulin-dependent diabetes mellitus: “EURODIAB IDDM Complications Study” – results from the Vienna center. Wiener Klinische Wochenschrift 1994;106:136–40.
- Kimmerle R, Heinemann L, Delecki A, Berger M. Severe hypoglycemia incidence and predisposing factors in 85 pregnancies of type I diabetic women. Diabetes Care 1992;15:1034–7.
- ADVANCE Collaborative Goup. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med 2008;358:2560–72.
- Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. New Engl J Med 2008;358:2545–59.
- Akram K, Pedersen-Bjergaard , Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycaemia in insulin-treated type 2 diabetes: A literature survey. J Diabet Complications 2006;20:402–8.
- Bell DS, Yumuk V. Frequency of severe hypoglycaemia in patients with non-insulin-dependent diabetes mellitus treated with sulfonylureas or insulin. Endocrine Practice 1997;3: 281–3.
- Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med 2001;161:1653–9.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53.
- UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: Effects of treatment modalities and their duration. Diabetologia 2007;50:1140–7.
- Honkasalo M, Elonheimo O, Sane T. HbA1c-määritysten tuloksissa on tasoeroa. (Variation in results of HbA1c determinations; English summary). Finnish Med J 2007;62:1609–12.
- Lloyd CE. Pambianco G. Orchard TJ. Does diabetes-related distress explain the presence of depressive symptoms and/or poor self-care in individuals with Type 1 diabetes? Diabet Med 2010;27:234–7.
- Heller S, Chapman J, McCloud J, Ward J. Unreliability of reports of hypoglycaemia by diabetic patients. BMJ 1995; 310:440.
- Mattila EM, Kuisma MJ, Sund KP, Voipio-Pulkki LM. Out-of-hospital hypoglycaemia is safely and cost-effectively treated by paramedics. Eur J Emergency Med 2004;11:70–4.
- Gough SC. A review of human and analogue insulin trials. Diabetes Res Clin Pract 2007;77:1–15.
- Pedersen-Bjergaard U, Pramming S, Heller SR, Wallace TM, Rasmussen AK, Jorgensen HV, . Severe hypoglycaemia in 1076 adult patients with type I diabetes: Influence of risk markers and selection. Diabetes Metab Res Rev 2004;20: 479–86.
- Ter Braak EW, Appelman AM, van de Laak M, Stolk RP, van Haeften TW, Erkelens DW. Clinical characteristics of type 1 diabetic patients with and without severe hypoglycemia. Diabetes Care 2000;23:1467–71.
- Miettola J, Niskanen LK, Viinamäki H, Kumpusalo E. Metabolic syndrome is associated with self-perceived depression. Scand J Prim Health Care 2008;26:203–10.
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: A meta-analysis. Diabetes Care 2008;31:2283–90.
- Paile-Hyvärinen M, Räikkönen K, Forsen T, Kajantie E, Yliharsila H, Salonen MK, . Depression and its association with diabetes, cardiovascular disease and birth weight. Ann Med 2007;39:634–40.
- Adolfsson ET, Smide B, Rosenblad A, Wikblad A. Does patient education facilitate diabetic patients’ possibilities to reach national treatment targets? A national survey in Swedish primary healthcare. Scand J Prim Health Care 2009;27:91–6.