Abstract
Objective. The view that the general practitioner (GP) should be more involved during the curative treatment of cancer is gaining support. This study aimed to assess the current role of the GP during treatment of patients with colorectal cancer (CRC). Design. Historical prospective study, using primary care data from two cohorts. Setting. Registration Network Groningen (RNG) consisting of 18 GPs in three group practices with a dynamic population of about 30 000 patients. Subjects. Patients who underwent curative treatment for CRC (n = 124) and matched primary care patients without CRC (reference population; n = 358). Main outcome measures. Primary healthcare use in the period 1998–2009. Findings. Patients with CRC had higher primary healthcare use in the year after diagnosis compared with the reference population. After correction for age, gender, and consultation behaviour, CRC patients had 54% (range 23–92%) more face-to-face contacts, 68% (range 36–108%) more drug prescriptions, and 35% (range –4–90%) more referrals compared with reference patients. Patients consulted their GP more often for reasons related to anaemia, abdominal pain, constipation, skin problems, and urinary infections. GPs also prescribed more acid reflux drugs, laxatives, anti-anaemic preparations, analgesics, and psycholeptics for CRC patients. Conclusions. The GP plays a significant role in the year after CRC diagnosis. This role may be associated with treatment-related side effects and psychological problems. Formal guidelines on the involvement of the GP during CRC treatment might ensure more effective allocation and communication of care between primary and secondary healthcare services.
This study is the first to analyse disease-specific primary health care use of patients treated with curative intent in the first year following diagnosis of colorectal cancer (CRC).
The general practitioner plays a significant role in the year after CRC diagnosis.
CRC patients show higher primary health care use compared with the reference population in the first year after diagnosis.
This higher health care use may be associated with treatment-related side effects and psychological problems.
Introduction
In countries where the general practitioner (GP) acts as a gatekeeper to the healthcare system, the GP traditionally plays an important role for patients with cancer: in early detection [Citation1,Citation2], managing comorbidities and psychosocial issues [Citation3], and palliative care [Citation4]. In the Netherlands, the expected increase in the prevalence of cancer is likely to impose a considerable burden on the healthcare system [Citation5]. According to the Dutch Cancer Society, this highlights the need for an effective resource allocation between primary care and secondary care [Citation6]. Internationally, there is increasing support for the idea that GPs (and other primary care providers) should be more involved in all stages of cancer care [Citation6–10].
In the Dutch guideline on colorectal cancer (CRC) [Citation11] no formal role is described for the GP during treatment. Moreover, healthcare use of CRC patients in primary care during treatment has not yet been studied. In a Dutch study on patients with breast cancer, increased contact rates with the GP were observed in the first year after diagnosis when compared with a reference population [Citation12]. Similar findings were reported in a study conducted in Denmark concerning all cancer types [Citation13], but the reasons associated with health care use were not examined in this study.
Treatment-related side effects of surgical treatment for CRC, chemotherapy, and radiotherapy may have a major impact on patients’ lives and perceived quality of life [Citation14,Citation15]. Whereas the role of the GP in diagnostic and referral pathways of CRC has been extensively studied [Citation2], little is known about the effect of CRC treatment and its side effects on patients’ healthcare use in primary care. Therefore, the aim of the current study is to assess the GP's role during curative treatment of patients with CRC by an analysis of health care use in the first year after diagnosis.
Material and methods
Design and setting
Healthcare use of patients during the first year after CRC diagnosis was compared with an age- and gender-matched reference population from the same general practice, in a historical prospective study. Healthcare utilization data were extracted from the database of the Registration Network Groningen (RNG). This GP registration network, founded in 1989, collects data from 18 GPs in three group practices in the northern part of the Netherlands with about 30 000 patients [Citation16]. In accordance with the privacy instructions of the RNG, anonymized patient records were used. Because the study was in agreement with the regulations for publication of routinely registered healthcare data, no further official approval was required.
Participants and data collection
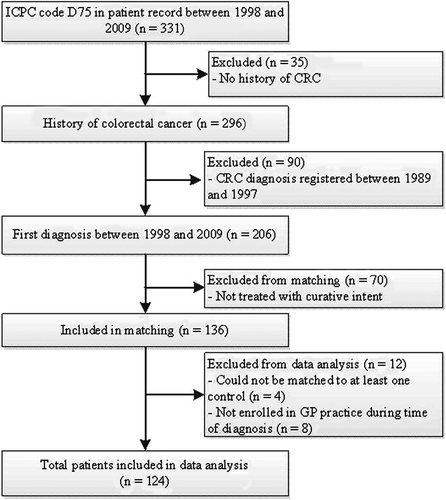
The study population was determined by selecting RNG patients with a first diagnosis of CRC between 1998 and 2009, using the D75 code (colon or rectum malignity) of the International Classification of Primary Care (ICPC) version 1 [Citation17]. The date of referral by the GP was used as the date of diagnosis. When unavailable, the first date of recording the D75 code (39.6% of cases) in the electronic patient record was used. The CRC diagnosis was validated in the GPs’ practices and additional information about the CRC stage, treatment, and recurrence was retrieved by examining specialist reports and hospital records. shows the process of identification and inclusion of study patients. Patients with a CRC diagnosis before 1998 (n = 90) were excluded because the recording of healthcare use by the RNG before 1998 was unavailable. Patients not treated with curative intent (n = 70) were also excluded, because palliative treatment of cancer is likely to generate excessive or atypical healthcare use in primary care [Citation18].
A reference population of patients without CRC was identified in the RNG database. Each CRC patient was individually matched (if possible) with three patients (with a minimum of one) on gender, age (± 1 year), and GP to control for recording and prescribing by GPs and for regional factors such as socioeconomic status. Four patients with CRC could not be matched with at least one patient from the reference population and were excluded. Also, eight patients and their references were excluded from data analysis because the patients were not registered at the GP's office at the time of diagnosis.
Finally, 124 patients with CRC and 358 reference patients were available for analysis. Data collected were entered in an anonymized database and consisted of patient contacts recorded by the GPs using ICPC codes, prescribed medication automatically classified according to the Anatomical Therapeutical Chemical (ATC) classification [Citation19], and referrals (in which the CRC referral was included).
Data analysis
Database management and statistical analyses were performed in Microsoft Access 2010 and IBM SPSS version 20, respectively. Due to the skewed distribution of the data, statistical analyses were performed using non-parametric tests and negative binomial regression analysis. To compare characteristics between patients and the reference population, the Mann–Whitney U-test was used.
Annual healthcare utilization rates in the year after diagnosis were calculated by dividing the number of face-to-face contacts, drug prescriptions, and referrals by the observation time. Face-to-face contacts consisted of consultations in general practice, as well as visits to patients’ homes made by GPs or other general practice workers. The annual healthcare utilization rates in the year prior to diagnosis were also calculated to allow for a comparison with utilization rates after diagnosis. Differences in median face-to-face contact, drug prescription, and referral rates between CRC patients and the reference population were analysed using the Mann–Whitney test. Wilcoxon's signed rank test was used to compare the healthcare use before and after diagnosis within the two groups.
To assess the size of the difference in healthcare use after diagnosis between CRC patients and the reference population, a multivariate negative binomial regression analysis was performed. To adjust for patients’ gender, age, and healthcare utilization rates before diagnosis, these variables were included in the model. Differences in observation time were accounted for by including the log-transformed observation time since diagnosis as an offset variable in the model.
A univariate negative binomial regression analysis was performed to evaluate whether specific characteristics of CRC patients (gender, age, TNM stage, and received therapy) were associated with higher rates of face-to-face contacts, prescribed medication, and referrals since diagnosis.
To examine reasons for primary healthcare use, numbers and percentages of CRC patients and the reference population with any face-to-face contact by ICPC code and any drug prescription by ATC code in the year after diagnosis were calculated. Differences in reasons for primary healthcare use between CRC patients and the reference population based on ICPC codes were tested with the Chi-square test.
Results
Characteristics of patients with CRC (n = 124) and the reference population (n = 358) are presented in . Median age at diagnosis was 69.3 (range 36.4–91.1) years.
Table I. Characteristics of the patients with colorectal cancer (n = 124) and the reference population (n = 358).
shows the annual healthcare utilization rates and numbers and percentages with at least one contact/prescription/referral of patients and the reference population in the period before and after CRC diagnosis. Patients had significantly more face-to-face contacts, prescriptions, and referrals compared with the reference population (Mann– Whitney test, p < 0.01) in both the year before and after diagnosis. Face-to-face contact rates and prescription rates showed a significant increase after diagnosis (Wilcoxon signed rank test, p < 0.01) in patients and in the reference population. Significantly more patients had at least one contact and referral before diagnosis, and significantly more patients had at least one contact, prescription, and referral after diagnosis (Chi-square test).
Table II. Annual healthcare utilization rates before and after diagnosis for patients with colorectal cancer (CRC) (n = 124) and the reference population (n = 358).
When corrected for age, gender, and healthcare use before diagnosis, results of the multiple negative binomial regression showed that patients had 54% more face-to-face contacts, 68% more drug prescriptions, and 35% more referrals in the year after diagnosis compared with the reference population ().
Table III. Rate ratios and 95% confidence intervals (CI) for face-to-face contacts, drug prescriptions, and referrals for patients with colorectal cancer in the year after diagnosis compared with the reference population.
Furthermore, univariate negative binomial regression analysis showed that only a higher age was associated with more face-to-face contacts after diagnosis. Higher rates of prescribed medication and referrals before diagnosis were associated with these rates after diagnosis. None of the other patient characteristics (as shown in ) were associated with higher healthcare use after diagnosis (data not shown).
presents the reasons for GP contacts and the prescribed medication in the year after diagnosis for CRC patients and the reference population. Significantly more patients contacted their GP for anaemia, abdominal pain, constipation, and urinary infections. Significantly more patients than those from the reference population were prescribed the following medication: acid reflux drugs and laxatives, mineral supplements, anti-anaemic preparations, analgesics, psycholeptics (mainly hypnotics and sedatives), and cough/cold preparations.
Table IV. Reasons for primary healthcare use among patients with colorectal cancer (CRC) (n = 124) and patients from the reference population (n = 358) in the year after diagnosis.
Discussion
This study explored the involvement of the GP during curative treatment of CRC. Patients showed higher healthcare use in primary care in the year after diagnosis compared with the reference population, and also compared with their healthcare use before diagnosis. Adjusted for gender, age, and healthcare use before diagnosis, patients had 54% more face-to-face contacts, 68% more drug prescriptions, and 35% more referrals after diagnosis compared with the reference population. Neither treatment nor tumour stage was associated with higher healthcare use. More patients consulted their GP for reasons related to anaemia, abdominal pain, constipation, skin problems, and urinary infections compared with the reference population. GPs also prescribed more acid reflux drugs, laxatives, anti-anaemic preparations, analgesics, and psycholeptics (mainly hypnotics and sedatives).
A major strength of this study is the use of a prospective primary care database to analyse and compare the healthcare use of patients with and without CRC. The presentation of healthcare problems was recorded by the GPs of the registration network, making recall or non-response bias less likely than in self-reported survey data [Citation20]. By matching on GP, any inaccuracies in recording and prescribing were evenly distributed among CRC patients and the reference population [Citation12]. Revalidating the data with specialist reports in GPs’ practices and available hospital records increased the likelihood that the patient information was correctly recorded by the GP.
In the Netherlands almost every inhabitant is registered with a GP [Citation21]. Therefore the RNG database is population-based. This population is comparable to the Dutch population in terms of gender and age with slightly more adults aged 25–44 and women [Citation22]. Nevertheless, care provided by the 18 GPs on the RNG might differ from other GPs in the Netherlands, possibly limiting the generalizability of the study results. Another limitation of the current study is the small number of patients, limiting the statistical power to find significant associations between patients’ characteristics and higher health care use. The finding that CRC patients have higher healthcare use in the year after diagnosis compared with the reference population is in line with an earlier Dutch study among patients with breast cancer and a Danish study among patients with all types of cancer [Citation12,Citation13]. Although not a focus of the present study, our findings show that CRC patients also have higher healthcare use during the year before diagnosis. This elevated healthcare use before diagnosis was not observed in patients with breast cancer [Citation12]. This might be due to the fact that, in contrast to breast cancer symptoms, most symptoms associated with CRC are common and relatively innocent, and because single symptoms have a low predictive value for CRC [Citation2]. In the Danish study an increase in primary healthcare use was also seen three months before diagnosis [Citation13]. Future research should further examine contact frequency and reasons associated with higher healthcare use in CRC patients in the period prior to diagnosis. The elevated healthcare use before diagnosis might also be explained by a higher prevalence of comorbidity in CRC patients.
According to our findings, received treatment and CRC staging were not associated with higher healthcare use in primary care after diagnosis. Problems associated with more aggressive treatments or more advanced cancer may be presented more in secondary care than in primary care.
The increased contact frequency for constipation, pain, micturition problems, and psychological problems might be related to the surgical therapy for CRC [Citation23–26]. The aforementioned reasons for GP contacts and prescribed medication may also be related to chemotherapy (often associated with adverse effects such as nausea and diarrhoea) [Citation27,Citation28] or to radiotherapy (rectal bleeding, pain, bowel and urinary problems) [Citation29,Citation30]. Comorbidity is known to affect healthcare use [Citation10]. However, analysis of the reasons for primary healthcare use based on the ICPC and ATC codes did not show increased healthcare use for chronic comorbidities, such as cardiovascular diseases, diabetes mellitus, and chronic obstructive pulmonary disease (COPD). Therefore, it is unlikely that the differences between the CRC patients and the reference population are due to differences in comorbidity.
Although no formal role has been established, the GP is involved in the treatment of problems associated with the side effects of curative CRC treatment. Furthermore, the GP is involved in the prescription of hypnotics and sedatives. The view that GPs and other primary care physicians should play a more formal role in all stages of cancer care is gaining support. Formal guidelines on the involvement of the GP during CRC treatment might ensure a more effective allocation and communication of care between primary and secondary healthcare services.
Acknowledgments
The authors thank the Stoffels-Hornstra Foundation for funding this study. They also thank the RNG for providing the data, and the participating general practitioners, practice assistants, and Marlieke Lohues for their help during data collection.
Source of funding
This study was supported by a grant from the Stoffels-Hornstra Foundation, Coevorden, the Netherlands. The funding source had no role in study design, data collection, analysis, and interpretation.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Summerton N. General practitioners and cancer. BMJ. 2000;320:1090–1.
- Astin M, Griffin T, Neal RD, Rose P, Hamilton W. The diagnostic value of symptoms for colorectal cancer in primary care: A systematic review. Br J Gen Pract. 2011;61(586):e231–43.
- Anvik T, Holtedahl KA, Mikalsen H.“When patients have cancer, they stop seeing me”: The role of the general practitioner in early follow-up of patients with cancer – a qualitative study. BMC Fam Pract. 2006;7:19–27.
- Johansen ML, Holtedahl KA, Rudebeck CE. A doctor close at hand: How GPs view their role in cancer care. Scand J Prim Health Care. 2010;28:249–55.
- Meulepas JM, Kiemeney LALM. In Meulepas JM, Kiemeney LALM. Kanker in Nederland tot 2020. Trends en prognoses [Cancer in the Netherlands till 2020. Trends and prognosis ]. Amsterdam: KWF Kankerbestrijding; 2011.
- Knottnerus A, Wijffels J.F.A.M. Nazorg bij kanker: de rol van de eerstelijn [Follow-up care for cancer: The role of primary care] Amsterdam: KWF kankerbestrijding; 2011.
- Grunfeld E. Cancer survivorship: A challenge for primary care physicians. Br J Gen Pract. 2005;55:741–2.
- McAvoy BR. General practitioners and cancer control. Med J Aust. 2007;187:115–17.
- Klabunde CN, Ambs A, Keating NL, He Y, Doucette WR, Tisnado D, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009;24:1029–36.
- Jabaaij L, van den Akker M, Schellevis FG. Excess of health care use in general practice and of comorbid chronic conditions in cancer patients compared to controls. BMC Fam Pract. 2012;13:60–6.
- IKNL. 9 January 2008. Landelijke richtlijn coloncarcinoom. Available at: http://www.oncoline.nl/coloncarcinoom (accessed 26 November 2013).
- Roorda C, de Bock GH, van der Veen WJ, Lindeman A, Jansen L, van der Meer K. Role of the general practitioner during the active breast cancer treatment phase: An analysis of health care use. Support Care Cancer. 2012;20:705–14.
- Christensen KG, Fenger-Gron M, Flarup KR, Vedsted P. Use of general practice, diagnostic investigations and hospital services before and after cancer diagnosis: A population-based nationwide registry study of 127,000 incident adult cancer patients. BMC Health Serv Res. 2012;12:224–30.
- Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: A population-based cross-sectional study. Colorectal Dis. 2013;15:1130–9.
- Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients – a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–206.
- Hiddema-van de Wal A, Smith RJ, van der Werf GT, Meyboom-de Jong B. Towards improvement of the accuracy and completeness of medication registration with the use of an electronic medical record (EMR). Fam Pract. 2001;18:288–91.
- Lamberts H, Wood M. ICPC. International classification of primary care. Oxford: Oxford University Press; 1987.
- Abarshi E, Echteld MA, Van den Block L, Donker G, Bossuyt N, Meeussen K, et al. Use of palliative care services and general practitioner visits at the end of life in The Netherlands and Belgium. J Pain Symptom Manage. 2011;41:436–48.
- WHO Collaborating Centre for Drug Statistics Methodology. ATC index with DDS. Oslo: WHO; 1999.
- Tisnado DM, Adams JL, Liu H, Damberg CL, Chen WP, Hu FA, et al. What is the concordance between the medical record and patient self-report as data sources for ambulatory care?Med Care. 2006;44:132–40.
- Knottnerus JA, ten Velden GH. Dutch doctors and their patients: Effects of health care reform in the Netherlands. N Engl J Med. 2007;357:2424–6.
- Van Wayenburg CA, van de Laar FA, de Waal MW, Okkes IM, van den Akker M, van der Veen WJ, et al. Nutritional deficiency in Dutch primary care: Data from general practice research and registration networks. Eur J Clin Nutr. 2005;59(Suppl 1):S187–94.
- Ho YH, Low D, Goh HS. Bowel function survey after segmental colorectal resections. Dis Colon Rectum. 1996; 39:307–10.
- Dunn J, Ng SK, Holland J, Aitken J, Youl P, Baade PD, et al. Trajectories of psychological distress after colorectal cancer. Psychooncology. 2013;22:1759–65.
- Breukink SO, Donovan KA. Physical and psychological effects of treatment on sexual functioning in colorectal cancer survivors. J Sex Med. 2013;10(Suppl 1):74–83.
- Theodoropoulos GE, Papanikolaou IG, Karantanos T, Zografos G. Post-colectomy assessment of gastrointestinal function: A prospective study on colorectal cancer patients. Tech Coloproctol. 2013;17:525–36.
- Lotfi-Jam K, Carey M, Jefford M, Schofield P, Charleson C, Aranda S. Nonpharmacologic strategies for managing common chemotherapy adverse effects: A systematic review. J Clin Oncol. 2008;26:5618–29.
- Jansman FG, Sleijfer DT, de Graaf JC, Coenen JL, Brouwers JR. Management of chemotherapy-induced adverse effects in the treatment of colorectal cancer. Drug Saf. 2001;24:353–67.
- Hayne D, Vaizey CJ, Boulos PB. Anorectal injury following pelvic radiotherapy. Br J Surg. 2001;88:1037–48.
- Adams E, Boulton MG, Horne A, Rose PW, Durrant L, Collingwood M, et al. The effects of pelvic radiotherapy on cancer survivors: Symptom profile, psychological morbidity and quality of life. Clin Oncol (R Coll Radiol). 2014;26:10–17. doi: 10.1016/j.clon.2013.08.003. Epub 2013 Aug 29.
Appendix: Overview of ICPC and ATC chapters.