To the Editor,
There is convincing evidence that adjuvant polychemotherapy improves survival in patients with early breast cancer. There are some conflicting studies on the prognostic significance of leukopenia during the adjuvant chemotherapy of early breast cancer. This prompted us to compare the outcome of adjuvant chemotherapy of early breast cancer patients correlated to nadir white cell counts.
One hundred and ninety-four women with either operable primary breast cancer or locoregional relapse were recruited from Eastern Finland. Staging was done by physical examination, radionuclide bone scan, liver ultrasound, chest x-ray, and laboratory examinations, including blood count, serum creatinine, and alkaline phosphatase. Peripheral blood counts and nadir counts were taken during and after the first chemotherapy cycle and routine blood counts one day before each successive treatment. Patients had undergone radical mastectomy, conservative surgery, or excision of a locoregional relapse. Postoperative radiotherapy was given to 191 patients. After surgery and radiotherapy the patients were administered CNF (cyclophosphamide 500 mg/m2, mitoxantrone 10 mg/m2, and 5-fluorouracil 500 mg/m2). Since February 1990, we replaced the first two chemotherapy cycles with CMF (methotrexate 40 mg/m2) in order to start chemotherapy simultaneously with the radiation therapy. After completing the chemo- and radiotherapy, patients were followed up every three months during the first year, and then once or twice a year for five years. In addition to ordinary follow-up, deaths and subsequent cancers were recorded from the files of the Finnish Cancer Registry.
Kaplan-Meier estimates for survival were calculated using corrected survival rates. Disease-free survival (DFS) was defined as the time from the first chemotherapy administration to detection of metastasis, locoregional relapse, death, or 15 January 2004. Only deaths due to breast cancer were regarded as events in the analysis of OS. Difference of survival curves between different levels of leukopenia was assessed by the log-rank and the Gehan-Wilcoxon tests. SPSS for Windows was used for the analyses.
The research plan was approved by the Finnish Ministry of Social and Health (permit Dnro 22/07/2003).
Women were pre-, peri- and postmenopausal aged 29 to 68 years. Primary breast cancer stages varied from I to IIIB. Nine patients were treated with locoregionally recurrent breast carcinoma. Of those patients with primary tumours, 174 patients were operated radically, 20 patients with conservative surgery followed by postoperative radiotherapy. Adjuvant hormonal therapy was prescribed to 50 patients (26%) beginning during the radiotherapy.
Adjuvant CMF/CNF chemotherapy was commenced from May 1986 to November 1993. The concomitant chemoradiation approach was started in February 1990, when the first two CNF courses were replaced with CMF. Eighty-seven patients (45%) received only CNF. All six courses of either CNF or CMF/CNF were administered to 173 patients (89%), and the mean number of courses was 5.8. The planned mitoxantrone dose was 10 mg/m2, but the actual dose ranged from 4.7 to 10.7 mg/m2 (median 8.9 mg/m2). The last mitoxantrone dose ranged from 3.5 to 10.7 mg/m2 (median 8.1 mg/m2). Twenty-one patients stopped chemotherapy prematurely for several non-fatal causes, the data of which has been published earlier Citation[1–3]. The patients were followed until 15 January 2004, and the median follow-up time was 12.9 years (range 10.2 to 17.7).
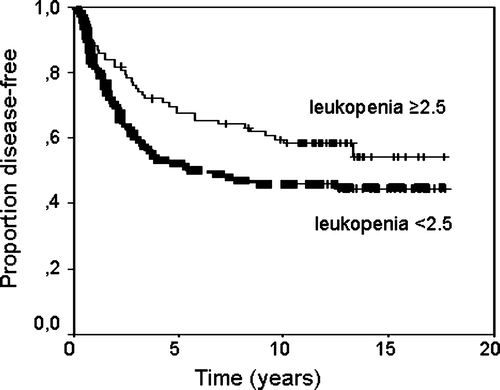
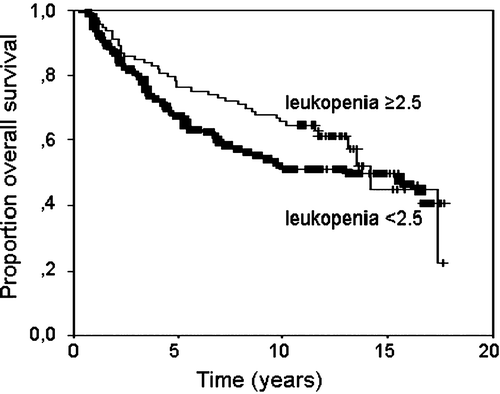
Grade 4 leukopenia (leukocyte count < 1.0×109/L) was documented in nine (5%) patients. The median nadir of leukocytes was 2.4. The patients were divided into two parts according to nadir leukocytes with lower leukocyte counts from 0.1 to 2.4, and higher leukocyte counts from 2.5 to 6.1×109/L. The ten year DFS was 45% for the lower, and 60% for the higher leukocyte patients, respectively (, p < 0.05). The ten year breast cancer specific survival was 51% and for the lower, and 66% for the higher leukocyte group (, p = 0.23). The Gehan-Wilcoxon test did not result in a change compared with the log-rank test (numbers not added).
This retrospective study showed that low nadir leukocyte count during adjuvant chemotherapy of breast cancer did not statistically significantly predict the prognosis. No rigid schedule was defined to reduce the chemotherapy doses occurring leukopenia, without compromising long-term outcome according to overall survival. Some searchers could make firm conclusions after five or ten year follow-up, but differences may disappear after longer follow-up, as in this setting. In this strategy, no multivariate analyses or dose intensity analyses were done, due to the number of patients and the versatility of prognostic factors.
The first report of dose-response effect by adjuvant CMF on relapses was published in 1981 Citation[4]. A subgroup of patients receiving less than 65% of the planned dose had a lower survival Citation[4].
Saarto et al. Citation[5] treated 211 stage II – III breast cancer patients giving eight doxorubicin-based cycles. They searched through initial dose intensities, and leukocyte nadir was a marker of chemotherapy efficacy in early breast cancer. Poikonen et al. Citation[6] treated 368 patients with stage II – III breast cancer giving six ordinary CMF courses. According to the univariate analyses, ordinary prognostic factors and low leucocyte nadirs were associated to both DFS and OS. However, leukocyte nadir lost the significance in the multivariate analysis. Canadian authors reviewed 680 patients with early breast cancer retrospectively Citation[7]. They suggested that myelosuppression improved survival. The dose intensity of classic oral CMF is higher than that of the intravenous regimen. They concluded that the classic regimen should be preferred due to better survival.
The Scandinavian Breast Group tested the effectiveness of very high dose adjuvant chemotherapy. Five hundred and twenty five breast cancer patients were randomized to receive either nine tailored CEF courses or three standard CEF doses and high-dose chemotherapy (cyclophosphamide, thiotepa, and carboplatin, with blood stem-cell support). Relapses of the patients of the latter therapy were higher, but up to eight acute leukemias and myelodysplastic syndromes developed in patients with the former therapy. High-dose chemotherapy did not improve overall survival Citation[8].
We failed to demonstrate a significant benefit for leukopenia of adjuvant chemotherapy for breast cancer patients.
Acknowledgements
This study was financially supported by the Finnish Breast Cancer Group, Kuopio University, and Kuopio University Hospital. I declare that I have no conflict of interest.
References
- Hirvikoski PP, Kumpulainen EJ, Johansson RT. CNF combination as adjuvant treatment in breast cancer patients is well tolerated. Anti-Cancer Drugs 1997; 8: 376–8
- Hirvikoski PP, Kumpulainen EJ, Johansson RT. Hepatic toxicity caused by adjuvant CMF/CNF in breast cancer patients and reversal by tamoxifen. Breast Cancer Res Treat 1997; 44: 269–74
- Kumpulainen EJ, Hirvikoski PP, Pukkala E, Johansson RT. Cancer risk after adjuvant chemo- or chemohormonal therapy of breast cancer. Anti-Cancer Drugs 1998; 9: 131–4
- Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. N Engl J Med 1981; 304: 10–5
- Saarto T, Blomqvist C, Rissanen P, Auvinen A, Elomaa I. Haematological toxicity: A marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br J Cancer 1997; 75: 301–5
- Poikonen P, Saarto T, Lundin J, Joensuu H, Blomqvist C. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer 1999; 80: 1763–6
- Mayers C, Panzarella T, Tannock IF. Analysis of the prognostic effects of inclusion in a clinical trial and of myelosuppression on survival after adjuvant chemotherapy for breast carcinoma. Cancer 2001; 91: 2246–57
- Bergh J, Wiklund T, Erikstein B, Lidbrink E, Lindmar H, Malmström P, et al. Tailored fluorouracil, epirubicin, and cyclophosphamide compared with marrow-supported high-dose chemotherapy as adjuvant treatment for high-risk breast cancer: A randomised trial. Scandinavian Breast Group 9401 study. Lancet 2000; 356: 1384–91