Abstract
Purpose. It is unknown to what extent lymph node metastases differ from primary tumours of breast cancer. Our aim was to investigate the similarity between primary breast tumours and the matching lymph node metastases in 59 breast cancer patients. Experimental design. Immunohistochemical stainings of p53, bax, bc-l2, fas and fasL were performed in primary tumours and the parallel lymph node metastases. Results. When using a cut point of 10%, the concordance between primary tumours and parallel lymph node metastases in the expression of p53 was 85%, bcl-2 79%, bax 69%, fas 59% and fasL 43%. In most tumours the staining status of p53, bcl-2 and bax in the primary tumour and the corresponding lymph node did not change more than 20%. However, these variables could fluctuate in both directions. In 15–25% of the cases, nodal expression was more than 20% lower than in the primary tumours, while in 10–17% of the cases, nodal expression was more than 20% higher than in the primary tumours. In half of the tumours, fas status did not change. Most fasL positive tumours lost positivity in the lymph node metastases or showed positively staining cancer cells only in the peripheral region of the node. A phenotype analysis of combined information of tumour fas/tumour fasL/nodal fas/nodal fasL expression (+/ − ) was assessed. The most frequently observed phenotype was tumour fas − /tumour fasL + /nodal fas − /nodal fasL− (22% of the tumours), although almost all combinations were seen. Conclusions. The expression of p53, bax, bcl − 2, fas and fasL is not maintained in the matching lymph node metastases of breast cancer. Large studies comparing the expression of relevant tumour biology factors in primary tumours and parallel lymph node metastases and their impact on therapy outcome, especially in the adjuvant setting, are warranted.
In breast cancer, the axillary lymph nodes are often the first site of metastases. Even though these regional metastases are not life-threatening, they are still the most powerful prognostic markers for distant metastases and death.
The biology of the multistep metastatic process is unclear Citation[1] and is most likely heterogenic, occurring either via lymph node metastasis or by the haematogenous route. It would be important to understand these heterogenic carcinogenic pathways, in order to develop novel, molecular-based targeted therapies. Previously, no tumour biologic factor has been identified to either discriminate lymph node metastases from primary breast tumours or to predict lymph node metastases of breast cancer. Surprisingly few reports have been published that compare lymph node metastases with primary breast tumours in parallel samples of patients.
In previous studies, estrogen receptor (ER) status, proliferation marker Ki-67 expression and HER-2 expression have been investigated in primary breast carcinomas and parallel axillary lymph node metastases. The concordance in ER status between primary tumours and lymph node metastases has been 80–90% Citation[2–4], while Ki-67 expression seems to be significantly higher in the axillary lymph node metastases than in the primary tumour Citation[5], Citation[6]. The concordance in HER-2 expression has been almost 100% in several studies Citation[7].
Tumours can arise either via excessive proliferation or via reduced cell death by apoptosis. Mitochondrial and cell surface death receptor-mediated apoptosis are the two principal pathways leading to programmed cell death Citation[8]. The mitochondrial pathway is mediated by over 20 members of the bcl-2 family proteins of which bcl-2 is an antiapoptotic protein and bax a proapoptotic protein. p53 is a transcription factor that participates in cell cycle checkpoint processes and apoptosis Citation[9].
The fas receptor (fas) belongs to the family of tumour necrosis factor (TNF) -related cell surface death receptors. Fas ligand (fasL) is its corresponding ligand which activates apoptosis by binding the death receptor. Both fas and fasL are widely expressed in normal human tissues Citation[10]. The co-expression of fas and fasL particularly in epithelia suggests that the physiological cell turnover of some tissues may be regulated by the fasR-fasL apoptotic pathway Citation[11]. Dysfunction of this system might allow tumour cells to escape beyond the physiological cell turnover. In addition, fas and fasL play a key role in the regulation of apoptosis within the immune system, which may in certain circumstances enable tumours to evade immune destruction by inducing apoptosis in activated lymphocytes near the tumour cells Citation[12]. Downregulation of fas has been observed in some carcinomas including breast cancer Citation[13], while fasL is sometimes overexpressed in many human tumours, including breast cancer Citation[14], Citation[15].
The purpose of the present study was to evaluate the expression of p53, bcl-2, bax, fas and fasL in the primary tumours as well as in the parallel lymph node metastases of patients with breast cancer.
Material and methods
Patients and therapy
Paraffin-embedded blocks of the primary tumour were available for 126 of 283 patients who took part in a randomised multicentre trial, comparing docetaxel to sequential methotrexate and 5-fluorouracil in advanced breast cancer Citation[16]. Of these 126 patients, 59 had lymph node metastases and paraffin-embedded blocks available for immunohistochemistry.
Immunohistochemical assays
All tissues had been fixed in 4% buffered formalin, processed and embedded in paraffin according to normal laboratory practice. From each block, 5 µm thick sections were cut onto coated slides and dried overnight at 37°C. The sections were deparaffinised in xylene and rehydrated through graded concentrations of ethanol in distilled water. Sections to be stained with antibodies against p53, bcl-2 and bax were pre-treated by boiling them in a microwave oven for 20 minutes in citrate buffer (pH 6.0). Those that were to be stained with anti-fas and anti-fasL were pre-treated by digestion in 0.5% trypsin (pH 7.2) at 37°C for 30 minutes. Immunohistochemical stainings were performed by using commercial Elite ABC Kits (Vectastain, Vector Laboratories, Burlingame, CA, USA). Blocking serum was applied for 15 minutes followed by overnight incubation with the diluted primary antibody p53 1:300 (clone D07, DAKO), bcl-2 1:200 (clone 124, DAKO), bax 1:100 (clone 2D2, Zymed), fas 1:250 (C20 rabbit polyclonal antibody raised against amino acids 316–335 mapping at the carboxy-terminus of the human FAS precursor, Santa Cruz) and fasL 1:100 (Q20 rabbit polyclonal antibody raised against a peptide corresponding to amino acids 2–21 mapping at the amino-terminus of FASL of human origin, Santa Cruz). The sections were then incubated with the biotinylated secondary antibody and the peroxidase-labelled ABC for 30 minutes each. All dilutions were made in phosphate buffered saline (PBS, pH 7.2), and all incubations were performed in humid chambers at room temperature. Between each step of the staining procedures, except before incubation with the primary antibody, the slides were rinsed three times in PBS. Bound peroxidase was visualised in all slides with a 3-amino-9-ethyl-carbazole (AEC) solution (Sigma) (0.2 mg/ml in 0.05M acetate buffer containing 0.03% perhydrol, pH 5.0) at room temperature for 15 minutes. Finally, the sections were lightly counterstained in Mayer′s hematoxylin and mounted in Aquamount Mountant (BDH Ltd, Poole, UK). For each antibody, a known positive case of breast cancer was included in every staining batch for positive control. The positive control sections for all antibodies were also stained with PBS instead of the primary antibody to exclude any false positivity. All staining batches contained a large number of sections so there were always negatively stained tumours among them which served as negative controls
Cells were considered positive only when distinct cellular micropunctate pattern of staining was seen, except for p53, for which nuclear staining was required. The percentage of immunoreactive cells was evaluated as the number of positive tumour cells per all the tumour cells on the section. All the stained sections were scored by two investigators (JS-M, ES) who were blinded to the clinical data. A cut point of 10% for positive expression was arbitrarily chosen for all the investigated tumour biologic factors except peripheral fasL (fasLp). We have shown previously that fasL staining is very often seen in the peripheral areas of the tumours Citation[17]. This is why we scored separately the peripheral staining of fasL (fasLp) in addition to the diffuse staining (fasL) in the lymph nodes. The nodal staining was considered positive for peripheral fasL if there were positively staining tumour cells in the peripheral regions of the metastases. The positively stained specimens were scored from 1 to 3 according to the number of positively stained cells, and scores 2–3 were considered as the cut point for positive peripheral expression.
Statistical methods
Spearman correlation coefficients were calculated for the investigated tumour biologic factors. Due to the high number of analyses, only p-values of equal to or less than 0.01 were considered significant. The correlations of the tumour biologic factors between the primary tumours and parallel lymph node metastases are also presented as scatter grams.
Results
Immunohistochemical stainings of p53, bcl-2, bax, fas and fasL were done both for the primary tumours and the corresponding lymph node metastases of the 59 breast cancer patients having lymph node metastases at the time of diagnosis. The characteristics of the 59 analysed tumours and parallel lymph nodes can be seen in . When using a cut point of 10%, the concordance between primary tumours and parallel lymph node metastases in the expression of p53 was 85%, bcl-2 79%, bax 69%, fas 59% and fasL 43%.
Table I. The characteristics of the primary tumours and parallel lymph node metastases at the time of diagnosis of the 59 investigated patients.
In addition to using an arbitrarily chosen cut point of 10%, we also analysed the results and made them meaningful by using a difference of 20% in the immunohistochemical assays between the primary tumours and corresponding lymph node metastases. In the majority of the tumours, the staining status of p53, bcl-2 and bax in the primary tumour and corresponding lymph node did not change more than 20% (). Changes in these variables between the primary tumours and their parallel metastases fluctuated in both directions. In 15–25% of the cases, nodal expression was more than 20% lower than in the primary tumours, while in 10–17% of the cases, nodal expression was more than 20% higher than in the primary tumours. In half of the tumours, fas status did not change. In most tumours, fasL status changed more than 20%, and in half of the cases it changed by losing its positivity in the lymph node metastases. In the fasL positive lymph nodes, the fasL staining was very often seen in the peripheral areas of the nodes (). This is why we scored separately the peripheral nodal staining of fasL (fasLp) and the diffuse nodal staining (fasL).
Figure 1. In the fasL positive lymph nodes the fasL staining was very often seen in the peripheral areas of the nodes. In this specimen the peripheral staining of fasL (fasLp) was scored as 3 and the diffuse nodal staining (fasL) as 20.
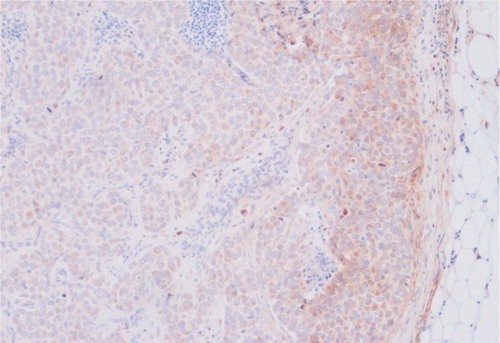
Table II. The change of the tumour biologic factors between primary tumours and parallel lymph node metastases.
The correlations between the investigated tumour biologic factors are shown in . Out of these factors, p53, bax and bcl-2 of the primary tumour correlated positively with the corresponding nodal expression. There was no statistically significant correlation between the fas or fasL status of the primary tumour and the corresponding lymph node metastases. p53 of the primary tumour was inversely correlated with nodal bcl-2, i.e. wild type p53 was associated with high expression of nodal bcl-2. Due to the large number of tests, only p ≤ 0.01 was considered significant.
Table III. The significance of the correlation (Spearman) between the investigated tumour biologic factors (n = 59 except for bax n = 58).
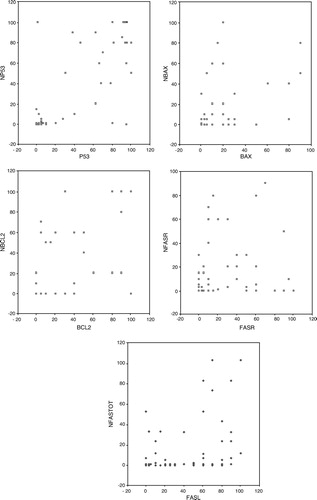
The correlations of the biologic factors between the primary tumours and parallel lymph node metastases are also presented as scatter grams in . In the scatter grams the exact percentages of the staining status oh both primary tumours and lymph node metastases can be seen in the same figure to avoid any impact of arbitrarily chosen cut points on the results.
Figure 2. The correlation of the tumour biologic factors between primary tumours and parallel lymph node metastases presented as scatter grams. x = percentage of positively staining cancer cells in the primary tumour, y = percentage of positively staining cancer cells in parallel lymph node metastasis.
A phenotype analysis of the combined information of tumour fas/tumour fasL/nodal fas/nodal fasL expression (+/ − ) was assessed (). Almost all combinations were seen. Most frequently seen phenotypes were tumour fas − /tumour fasL + /nodal fas − /nodal fasL− (22% of the tumours), as well as tumour fas + /tumour fasL + /nodal fas + /nodal fasL+ and tumour fas + /tumour fasL + / nodal fas − /nodal fasL+ (15% of the tumours).
Table IV. The combined status of fas and fasL of the primary tumour and parallel lymph node metastases. Here nodal fasL positivity (+) means that nodal fasL ≥ 10% and/or nodal peripheral fasL ≥ 2.
Discussion
Our data show that the expression of p53, bax, bcl-2, fas and fasL is not maintained in the matching lymph node metastases of breast cancer. Subtle, dynamic changes that happen all the time in the tumour, due to its crosstalk with surrounding environment and ongoing carcinogenesis, might explain the differences between the primary tumour and the counterpart lymph node metastases. Of the investigated factors, p53 status was maintained best, most likely because its expressional level is mainly a consequence of genomic mutations. The discrepancy found also in p53 status could be partly explained by the immunohistochemical method that was used, instead of the genomic assays. In addition to other groups, we have previously shown in this same patient material that HER-2 receptor, which is also a product of an oncogene, is so far the only tumour biologic factor that is generally expressed in lymph node metastases to the same extent as in the corresponding primary breast tumours Citation[7].
Although the findings of many earlier studies support the clonal theory of metastases, no intracellular molecular factor necessary for breast carcinoma metastases to occur has been identified. In fact, Weigelt et al. could not identify any classifier or single gene discriminating the group of primary breast tumours from those of the lymph node metastases. However, they did find subtle differences in gene expression profiles of different sets of genes involved in extracellular matrix organisation and growth factor signalling in primary breast tumours and their counterpart lymph node metastases Citation[18]. These genes were either up- or downregulated in lymph node metastases. Indeed, recent data suggest that metastatic dissemination does not depend on the acquisition of additional genetic lesions beyond those that are present in many primary tumours, but that it would rather occur as an inadvertent side effect of primary tumour formation Citation[19]. Nevertheless, increased mutability of tumour cells would still result in random genetic background and heterogeneity between primary tumours and metastases.
Only Iochim et al. have investigated fasL and fas expression in the nodal specimens of breast cancer. They also found equal or less fasL expression in the lymph node metastases than in the primary tumour counterparts, which correlates with our results Citation[20]. Additionally, they reported fas expression equal to or stronger than fasL in the primary mammary tumours and the reversal of their expression (i.e. fasL greater than fas) in the lymph node metastases. In the present study, fasL expression was lost in most lymph node metastases and we could not detect any positive statistical correlation between the primary tumour expression and nodal expression of fas or fasL. We detected several phenotypes of the combined status of fas and fasL of the primary tumour and parallel lymph node metastases, which probably indicates their interaction with the extracellular milieu rather than causing the metastases to occur. All these results might suggest that nodal fas/fasL system is most likely not a crucial, but rather a contributing or only a reactive factor in the complex process of breast cancer metastases.
In conclusion, the expression of p53, bax, bcl-2, fas and fasL is not maintained in the matching lymph node metastases of breast cancer. According to the literature, the only tumour biologic factor that has been reported to be expressed in lymph node metastases to the same extent as in the corresponding primary breast tumours is still HER-2.
In the future, we should probably make tumour biologic assays in addition to primary tumours also of lymph node metastases as well as of distant metastases in order to tailor the therapy optimally, since primary tumours and the corresponding metastases seem to differ in many respects. Large studies comparing the expression of relevant tumour biology factors in primary tumours and parallel lymph node metastases and their impact on therapy outcome, especially in the adjuvant setting, are warranted.
Acknowledgements
We thank the departments of pathology of the participating institutions for providing us with tumour specimens, Prof. Eero Saksela for consultation in scoring the tumours and Ms. Helena Huotarinen and Ms. Elina Laitinen for technical assistance. The study was supported by a state subsidy for research and development to Helsinki University Central Hospital. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–72
- Andersen J, Poulsen HS. Relationship between estrogen receptor status in the primary tumour and its regional and distant metastases. An immunohistochemical study in human breast cancer. Acta Oncol 1988; 27: 761–5
- Kamby C, Rasmussen BB, Kristensen B. Oestrogen receptor status of primary breast carcinomas and their metastases. Relation to pattern of spread and survival after recurrence. Br J Cancer 1989; 60: 252–7
- Nedergaard L, Haerslev T, Jacobsen GK. Immunohistochemical study of estrogen receptors in primary breast carcinomas and their lymph node metastases including comparison of two monoclonal antibodies. APMIS. 1995; 103: 20–4
- Buxant F, Anaf V, Simon P, Fayt I, Noel JC. Ki-67 immunostaining activity is higher in positive axillary lymph nodes than in the primary breast tumor. Breast Cancer Res Treat 2002; 75: 1–3
- Park D, Karesen R, Noren T, Sauer T. Ki-67 expression in primary breast carcinomas and their axillary lymph node metastases: Clinical implications. Virchows Arch 2007; 451: 11–8
- Carlsson J, Nordgren H, Sjostrom J, Wester K, Villman K, Bengtsson NO, et al. HER2 expression in breast cancer primary tumours and corresponding metastases. Original data and literature review. Br J Cancer 2004; 90: 2344–8
- Reed JC. Mechanisms of apoptosis. Am J Pathol 2000; 157: 1415–30
- el-Deiry WS. Regulation of p53 downstream genes. Semin Cancer Biol 1998; 8: 345–57
- Lee SH, Shin MS, Park WS, Kim SY, Dong SM, Lee HK, et al. Immunohistochemical analysis of fas ligand expression in normal human tissues. APMIS 1999; 107: 1013–9
- Xerri L, Devilard E, Hassoun J, Mawas C, Birg F. Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with fas in various epithelial tissues. Mol Pathol 1997; 50: 87–91
- Igney FH, Krammer PH. Tumor counterattack: Fact or fiction?. Cancer Immunol Immunother 2005; 54: 1127–36
- Keane MM, Ettenberg SA, Lowrey GA, Russell EK, Lipkowitz S. Fas expression and function in normal and malignant breast cell lines. Cancer Res 1996; 56: 4791–8
- Herrnring C, Reimer T, Jeschke U, Makovitzky J, Kruger K, Gerber B, et al. Expression of the apoptosis-inducing ligands FasL and TRAIL in malignant and benign human breast tumors. Histochem Cell Biol 2000; 113: 189–94
- Mullauer L, Mosberger I, Grusch M, Rudas M, Chott A. Fas ligand is expressed in normal breast epithelial cells and is frequently up-regulated in breast cancer. J Pathol 2000; 190: 20–30
- Sjöström J, Blomqvist C, Mouridsen H, Pluzanska A, Ottosson-Lonn S, Bengtsson NO, et al. Docetaxel compared with sequential methotrexate and 5-fluorouracil in patients with advanced breast cancer after anthracycline failure: A randomised phase III study with crossover on progression by the Scandinavian Breast Group. Eur J Cancer 1999; 35: 1194–201
- Sjöström J, Blomqvist C, von Boguslawski K, Bengtsson NO, Mjaaland I, Malmstrom P, et al. The predictive value of bcl-2, bax, bcl-xL, bag-1, fas, and fasL for chemotherapy response in advanced breast cancer. Clin Cancer Res 2002; 8: 811–6
- Weigelt B, Wessels LF, Bosma AJ, Glas AM, Nuyten DS, He YD, et al. No common denominator for breast cancer lymph node metastasis. Br J Cancer 2005; 93: 924–32
- Weinberg RA. Mechanisms of malignant progression. Carcinogenesis 2008; 29: 1092–5
- Ioachim HL, Decuseara R, Giancotti F, Dorsett BH. FAS and FAS-L expression by tumor cells and lymphocytes in breast carcinomas and their lymph node metastases. Pathol Res Pract 2005; 200: 743–51