Abstract
Background. Exosomes are nanometer-sized vesicles with immunomodulatory functions, which are released by a diverse range of living cells. Although recent studies have shown that tumor-derived exosomes can suppress the function of T cells, the molecular mechanisms are not well understood. In the present study, we investigated the role of the Casitas B lineage lymphoma (cbl) family of ubiquitin ligases in gastric cancer exosome-induced apoptosis of Jurkat T cells. Materials and methods. By serial centrifugation and sucrose gradient ultracentrifugation, we isolated and purified the exosomes from gastric cancer SGC7901 cells, and identified them by electron microscopy and Western blotting. Cell apoptosis was detected using propidium iodide staining. Western blotting and RT-PCR was exploited to evaluate the expression of proteins and mRNA, respectively. Results. Gastric cancer exosomes induced Jurkat T cell apoptosis in a time- and dose-dependent manner and activated caspases 3, 8 and 9. The expression of Cbl-b and c-Cbl was up-regulated during exosome-induced apoptosis of cells. Meanwhile, exosomes induced ubiquitination of the p85 subunit of phosphoinositide 3-kinase (PI3K) and reduced downstream Akt activity. Inhibition of proteasome led to partial restoration of Akt activity and cell apoptosis. Discussion and Conclusions. The Cbl family of ubiquitin ligases might be involved in regulation of exosome-induced apoptosis of Jurkat T cells by increasing PI3K proteasome degradation, inactivation of PI3K/Akt signaling, thus mediating some effects of caspase activation.
Tumors are known to escape successfully from the host immune system by several mechanisms, one of which is the acquisition of Fas ligand (FasL) and/or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) expression, which may enable cancer cells to deliver death signals to activated T cells Citation[1]. A recent study has shown that many cancer cells can release FasL- and TRAIL-bearing exosomes both in vitro and in vivo, which may account for the apoptosis of activated T cells and enhanced tumor cell survival Citation[2–4].
Exosomes are nanometer-sized membrane vesicles that accumulate intracellularly into late or multivesicular endosomes, and are released extracellularly after fusion of multivesicular endosomes with the cell membrane Citation[5]. Many tumor cells, as well as hemopoietic and epithelial cells, produce exosomes Citation[5–7], yet, their function remains enigmatic Citation[8]. Much of the prior work has focused on exosomes as a novel cell-free source of tumor antigens for the development of more effective immunotherapeutic strategies Citation[9], Citation[10]. However, recent evidence has shown that tumor-derived exosomes may also exert a broad array of detrimental effects on the immune system, including blocking of signaling and inducing apoptosis of activated T cells. While emerging evidence has suggested that Casitas B lineage lymphoma-b (Cbl-b), the E3 ubiquitin ligases, is responsible for activation-induced apoptosis in murine T helper 1 (Th1) cells following CD3 ligation Citation[11], it is uncertain whether cbl proteins possess the ability to regulate exosome-induced apoptosis of T cells.
The Cbl family of proteins, including Cbl-b and c-Cbl, are members of a superfamily of RING finger E3 ubiquitin ligases. The ubiquitination of proteins by E3 ligases has recently been recognized as an important regulatory mechanism for a variety of immune functions, such as maintenance of T-cell homeostasis and self tolerance Citation[12], Citation[13]. Cbl-b and c-Cbl not only play an essential role in setting a signaling threshold for T-cell activation, but also target tyrosine kinase receptors and growth signaling proteins, which results in their ubiquitination and down-regulation Citation[12]. Among these proteins, Cbl proteins can interact with the p85-regulatory subunit of phosphoinositide 3-kinase (PI3K), which leads to PI3K ubiquitination and degradation Citation[12], Citation[14]. PI3K catalyzes the production of phosphatidylinositol-3,4,5-trisphosphate and activates the downstream Akt, and therefore contributes to the activation of various signaling components involved in the regulation of gene expression and cell survival Citation[15]. Several studies have shown that T cells expressing active Akt are resistant to activation-induced apoptosis in vitro and in vivoCitation[16], Citation[17]. However, it remains unknown whether tumor-derived exosomes affect the PI3K/Akt pathway during apoptosis of T cells.
In the present study, we evaluated the role of the Cbl family of ubiquitin ligases in gastric cancer exosome-induced apoptosis of Jurkat T cells. The results demonstrated that the expression of Cbl-b and c-Cbl was up-regulated, which was accompanied by ubiquitination of the p85 subunit of PI3K and inhibition of Akt activity, during exosome-induced apoptosis. Inhibition of proteasome led to partial restoration of Akt activity and cell apoptosis. This study delineated a new regulatory function for Cbl-proteasome pathway in tumor immunity.
Materials and methods
Materials and antibodies
Rabbit anti-caspases 8 and mouse anti-caspase 9 antibodies were purchased from Lab Vision Corporation (Fremont, CA). Rabbit anti-Akt and phospho-Akt antibodies were purchased from Cell Signaling Technology (Danvers, MA). Goat anti-HLA-A, rabbit anti-caspase 3, mouse anti-CD9, anti-Cbl-b and anti-ubiquitin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-c-Cbl monoclonal antibody was purchased from Transduction Laboratories (Lexington, KY). Mouse anti-tubulin monoclonal antibody was purchased from BD Biosciences Pharmingen (San Jose, CA). Mouse anti-p85 antibody was purchased from UBI (Lake Placid, NY). The proteasome inhibitor PS-341 was purchased from Ben Venue Laboratories Inc. (Bedford, OH).
Cells and cell culture
Human gastric adenocarcinoma SGC7901 cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Jurkat T cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD). SGC7901 and Jutkat T cells were cultured in RPMI 1640 medium that contained 10% heat-inactivated fetal bovine serum (FBS), penicillin (5 U/ml) and streptomycin (50 mg/ml) under a 95% air/5% CO2 atmosphere. FBS was predepleted of bovine exosomes by ultracentrifugation at 100 000×g for 16 h at 4°C.
Exosome purification
Exosomes were purified from the 48 h supernatants of 80% confluent SGC7901 cells, using a modification of the serial centrifugation and sucrose gradient ultracentrifugation procedure described by Lamparski et al. Citation[18]. Briefly, separation of cellular debris was performed by centrifugation at 1000×g for 10 min. The clarified supernatant was concentrated by centrifugation for 30 min at 1000×g in a pre-rinsed 100-kDa MWCO hollow fiber membrane (Millipore, Bedford, MA). The concentrated exosomes were collected and transferred to an ultracentrifuge tube. The exosomes were underlayed with a 30% sucrose/D2O density cushion, followed by ultracentrifugation (SORVALL ULTRA Pro80; Kendro, Newtown, CT) in a Surespin 630 swinging bucket at 100 000×g and 4°C for 1 h. At the bottom, the cushion was collected and washed three times with phosphate-buffered saline (PBS) by centrifuging for 30 min at 1000×g in a pre-rinsed 100-kDa MWCO Millipore Ultrafree-15 capsule. The protein concentration of exosomes was assessed by Bradford assay (Bio-Rad, Hercules, CA).
Electron microscopy
Exosomes obtained after differential ultracentrifugation were fixed in 4% paraformaldehyde and stored at 4°C. Then, exosomes were loaded onto electron-microscopy grids coated with formvar carbon, contrasted and embedded them in a mixture of uranyl acetate and methylcellulose. Sections were viewed in a JEM-1200EX transmission electron microscope (JEOL, Japan). Exosome size was measured by the scale bar.
Apoptosis detection by propidium iodide (PI) staining
Jurkat T cells (3×105/ml) were treated with the indicated concentrations of exosomes for 24 and 48 h. Then, the cells were collected and washed twice with ice-cold PBS and fixed with ice-cold 70% ethanol overnight at 4°C. After removing the fixing solution, the sample were washed twice with PBS and incubated with 10 mg/ml PI at room temperature for 30 min in the dark. Finally, samples were evaluated by flow cytometry and data were analysed using CellQuest (FACS Calibur, Becton Dickinson, Franklin Lakes, NJ). To assess the effect of Cbl family of ubiquitin ligases on cell apoptosis, Jurkat T cells were pretreated with or without 10 nM PS-341 for 4 h, followed by the addition of 400 µg/ml exosomes.
Western blotting and immunoprecipitation
Jurkat T cells either treated or untreated with exosomes were cultured and harvested at the indicated time. Cell pellets were washed twice with ice-cold PBS and solubilized in 1% Triton lysis buffer (1% Triton X-100, 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 10 mM EDTA, 100 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, and 90 mU/ml aprotinin) on ice. For immunoprecipitation, cells were solubilized in the denaturation buffer (1% Triton X-100 lysis buffer containing 0.1% sodium dodecylsulfate [SDS] and 0.5% deoxycholic acid) to dissociate protein complexes. Cell lysates were mixed with p85 antibody at 4°C overnight followed by the addition of 30 µl of protein G-Sepharose beads (Santa Cruz Biotechnology) for an additional 2 h at 4°C. The immunoprecipitated proteins were eluted by heat treatment at 100°C for 5 min with 2×sampling buffer. For the preparation of total cell lysates, cells were washed as described earlier with ice-cold PBS and lysed with 1% Triton lysis buffer, followed by addition of 3× sampling buffer. Cell lysates and immunoprecipitated proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electronically transferred to nitrocellulose membranes. After blocking with 5% skim milk in TBST (10 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween 20), the blots were probed with the indicated primary antibodies at 4°C overnight, followed by the horseradish peroxidase-conjugated specific goat anti-mouse or goat anti-rabbit or rabbit anti-goat secondary antibody as indicated for 30 min at room temperature. Finally proteins were visualized by the enhanced chemiluminescence reagent (SuperSignal Western Pico Chemilunescent Substrate; Pierce, Rockford, IL). The final result was analyzed by NIH Image J software.
For Western blotting analysis of exosome protein expression, exosome or SGC 7901 cell lysate proteins were extracted as described previously Citation[19]. Equal amount of proteins from exosome preparation or total cell lysates (20 µg) were applied to each lane of a 10% polyacrylamide gel, as described above with anti-human HLA-A and CD9 antibodies. We also detected the expression of Cbl-b, c-Cbl, caspase 3, 8 and 9 on exosomes.
Reverse-transcription-polymerase chain reaction (RT-PCR)
Jurkat T cells either treated or untreated with exosomes were cultured and harvested at the indicated time. Cell pellets were washed twice with ice-cold PBS and total RNA extracted with the RNeasy mini kit (Qiagen, Carlsbad, CA) as described by the manufacturer. PT-PCR was performed with primer pairs for c-Cbl: forward (5′-CCTGGCAGTTATATCTTCCGG) and reverse (5′-TACCACGATGGGTTCAGTACC), for Cbl-b: forward (5′-CCGGTTAAGTTGCACTCGAT) and reverse (5′-CAAAGGGGTCCACGATTATG), and for β-actin as a control: forward (5′-GTGGGGCGCCCCAGGCACCA) and reverse (5′-CTCCTTAATGTCACGCACGATTTC). For c-Cbl, PCR conditions were 94°C for 5 min; 35 cycles of 94°C for 30 s, 57°C for 30 s, 72°C for 30 s; one cycle of 72°C for 10 min. For Cbl-b, PCR conditions were 95°C for 5 min; 30 cycles of 95°C for 30 s, 59°C for 30 s, and 72°C for 30 s; one cycle at 72°C for 7 min. For β-actin, PCR conditions were 94°C for 5 min; 30 cycles of 94°C for 1 min, 60°C for 1 min, 72°C for 1.5 min; one cycle of 72°C for 7 min. The amplified products were then separated on 1.5% agarose gels, stained with ethidium bromide, and visualized under UV illumination.
Statistical analysis
All experiments were repeated at least three times. Data were expressed as mean±standard deviation (SD). Statistical significance was determined by Student's t-test. All tests were one-sided and p < 0.05 was considered to be statistically significant. Analyses were conducted with SPSS13.0 software.
Results
Identification of tumor-derived exosomes
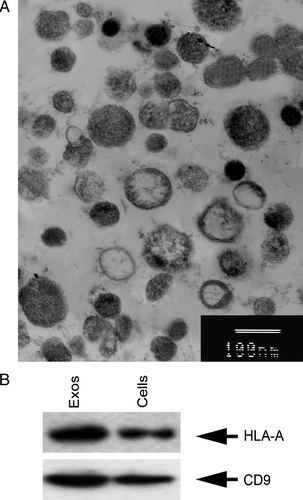
By serial centrifugation and sucrose gradient ultracentrifugation, exosomes were isolated and purified from SGC7901 cells. Electron microscopy showed that the exosomes had a characteristic saucer-like shape that was limited by a lipid bilayer, and their diameter ranged from 30 to 100 nm (A). It has been demonstrated that tumor-derived exosomes are rich in molecules involved in antigen presentation, such as MHC class I and tetraspanin molecules Citation[9]. The present study detected the molecular phenotype of the exosomes and showed that SGC7901-cell-derived exosomes stained positively for HLA-A antigens and CD9 (B).
Figure 1. Identification of tumor-derived exosomes. The exosomes were isolated and purified from SGC7901 cells, and visualized under electron microscopy (A). They had saucer-like vesicles limited by a bilayer membrane, with a size of 30–100 nm. Bars, 100 nm. (B) Western blotting analysis of protein composition of exosomes. Equal amounts of exosome protein derived from SGC7901 cells and lysates were immunoblotted with anti-human HLA-A and CD9 antibodies.
Induction of apoptosis by tumor-derived exosomes
Viable Jurkat T cells were incubated in medium supplemented with exosomes at the indicated concentrations for 24 and 48 h. Flow cytometry showed that a time- and dose-dependent induction of apoptosis was observed in Jurkat T cells treated with exosomes (A and B). After treatment with 400 µg/ml exosomes for 48 h, 31.76% of the cells underwent apoptosis, while only 2.47% of the control cells underwent apoptosis (p < 0.01). To identify the mechanisms of exosome-induced apoptosis, we examined the levels of proteins related to apoptosis. Treatment with 400 µg/ml exosomes led to the activation of caspases 3, 8 and 9 (A and B). To rule out the possibility that the appearance of cleaved caspases resulted from exosomes added to the cells, we detected the expression of caspases on exosomes, and showed that SGC7901-cell-derived exosomes stained negatively for caspases 3, 8 and 9 (data not shown).
Figure 2. Tumor-derived exosomes induced Jurkat T cell apoptosis. (A) Jurkat T cells (3×105 cells/ml) were exposed to 0, 25, 100 and 400 µg/ml exosomes for 24 and 48 h, and cell apoptosis was analyzed by flow cytometry following staining with PI. Data are the mean±SD of at least three independent experiments. N.S. indicates no significant differences; * p < 0.05; ** p < 0.01. (B) Jurkat T cells were treated with the indicated concentrations of exosomes for 48 h, and cell apoptosis was analyzed by flow cytometry. Data are representative of one of three independent experiments.
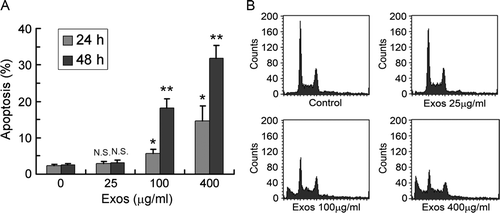
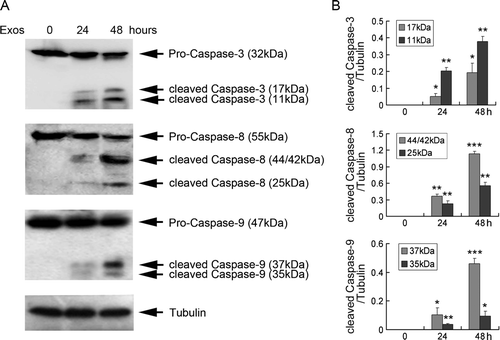
Figure 3. Exosomes induced caspase activation in Jurkat T cells. (A) Jurkat T cells were exposed to 400 µg/ml exosomes for 24 and 48 h, and the expression of caspases 3, 8 and 9 was analyzed by Western blotting. Tubulin was used as an internal control. Data are representative of one of three independent experiments. (B) The levels of caspases 3, 8 and 9 were measured by NIH Image J software and corrected for tubulin. Data are the mean±SD of three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
Up-regulated expression of the Cbl ubiquitin ligases in exosome-treated Jurkat T cells
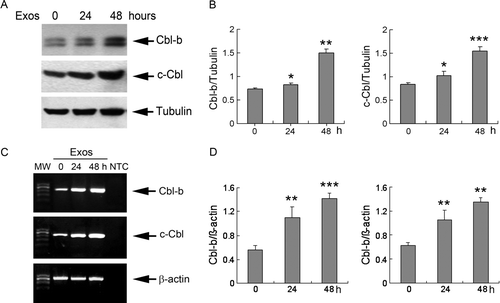
After Jutakt T cells were treated with exosomes at 400 µg/ml for 24 and 48 h, total cell lysates showed increased expression of Cbl-b and c-Cbl (A and B). The protein expression of Cbl-b and c-Cbl was gradually up-regulated at 24 h (1.10- and 1.23-fold versus controls, respectively), and increased sharply at 48 h (2.04- and 1.86-fold versus controls, respectively). RT-PCR analysis showed up-regulation of Cbl-b and c-Cbl mRNA after treatment with exosomes (C and D) that correlated with the increase of Cbl-b and c-Cbl proteins. To rule out the possibility that the increased expression of Cbl-b and c-Cbl proteins resulted from exosomes added to the cells, we detected the expression of Cbl-b and c-Cbl on exosomes, and showed that SGC7901-cell-derived exosomes stained negatively for Cbl-b and c-Cbl (data not shown). These results indicated that exosome-induced apoptosis of Jurkat T cells was accompanied by an increase in Cbl protein synthesis and expression.
Figure 4. Exosomes up-regulated expression of Cbl ubiquitin ligases. (A) Jurkat T cells were exposed to 400 µg/ml exosomes for 24 and 48 h, and the protein expression of Cbl-b and c-Cbl was analyzed by Western blotting. Tubulin was used as an internal control. (B) The levels of Cbl-b and c-Cbl were measured by NIH Image J software and corrected for tubulin. (C) Jurkat T cells were treated with exosomes as described above, and the expression of mRNA for Cbl-b and c-Cbl was analyzed by RT-PCR. β-actin was used as an internal control. MW, molecular weight; NTC, no template control reaction. (D) The levels of Cbl-b and c-Cbl were measured by NIH Image J software and corrected for β-actin. Data are the mean±SD of three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
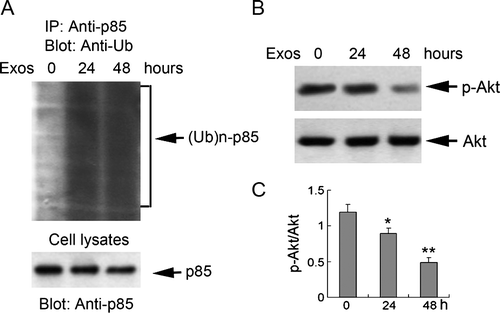
Induction of p85 ubiquitination and decreased downstream Akt activity by exosomes
Classical PI3K is composed of a p85 regulatory subunit and a p110 catalytic subunit. Since Cbl proteins can interact with p85, which leads to PI3K ubiquitination and degradation Citation[14], we examined whether p85 ubiquitination was induced by exosomes. Immunoprecipitation analysis showed that treatment with exosomes led to the appearance of p85 subunit polyubiquitination (A). Furthermore, the study detected the activity of Akt, and the expression of p-Akt was gradually down-regulated at 24 h (0.75-fold versus control) and decreased sharply at 48 h (0.41-fold versus control) (B and C), which coincided with the increased expression of Cbl-b and c-Cbl. These results indicated that exosome-induced apoptosis of Jurkat T cells was accompanied by p85 subunit polyubiquitination and a decrease in Akt activity.
Figure 5. Exosomes induced ubiquitination of the p85 subunit of PI3K and reduced downstream Akt activity in Jurkat T cells. Jurkat T cells were exposed to 400 µg/ml exosomes for 24 and 48 h. (A) Cell lysates were precipitated with anti-p85 agarose, and analyzed by Western blotting with anti-ubiquitin antibody. An aliquot of the cell lysates was immunoblotted with anti-p85 antibody to detect p85 expression (B) The expression of phosphorylated (p)-Akt and Akt were analyzed by Western blotting. (C) The levels of p-Akt were measured by NIH Image J software and corrected for Akt. Data are the mean±SD of three independent experiments. * p < 0.05; ** p < 0.01; *** p < 0.001.
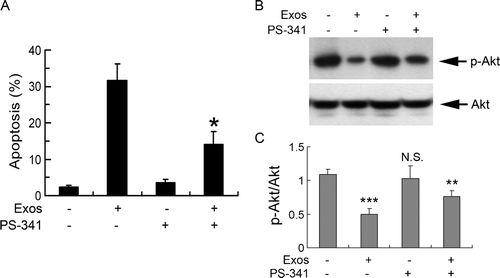
Role of Cbl family of ubiquitin ligase in exosome-induced apoptosis of Jurkat T cells
We explored whether the up-regulation of Cbl proteins was involved in cell apoptosis. PS-341, the proteasome inhibitor was used to block the function of Cbl proteins, and cell apoptosis was determined. Pretreatment with 10 nM PS-341 for 4 h partially reversed exosome-induced apoptosis (A). At the same time, up-regulation of phosphorylated Akt was also partially restored by pretreatment with PS-341 (B and C). These effects were not due to non-specific effects of PS-341 since cell apoptosis was not affected by 10 nM PS-341 treatment alone (A), which indicated that Cbl proteins and downstream PI3K/Akt signaling might be involved in exosome-induced apoptosis.
Figure 6. Role of Cbl family of ubiquitin ligases in exosome-induced apoptosis of Jurkat T cells. (A) Jurkat T cells were exposed to 400 µg/ml exosomes for 48 h in the absence or presence of 10 nM PS-341, and cell apoptosis was analyzed by flow cytometry following staining with PI. * p < 0.05 vs. exosomes alone. Data are the mean±SD of three independent experiments. (B) Jurkat T cells were exposed to 400 µg/ml exosomes for 48 h in the absence or presence of 10 nM PS-341 and the cell lysates were analyzed by Western blotting using antibodies specific for p-Akt and Akt. (C) The levels of p-Akt were measured by NIH Image J software and corrected for Akt. Data are the mean±SD of three independent experiments. N.S. indicates no significant differences; * p < 0.05; ** p < 0.01; *** p < 0.001.
Discussion
Despite the great progress made in developing immunotherapeutic interventions, little clinical success has been achieved. This might be because of the profound immune defects found in advanced cancer patients. It has been reported recently that tumor-derived exosomes are responsible for defective signaling responses in T cells, by down-modulating CD3-ζ and Janus-activated kinase 3, which results in apoptosis Citation[4]. Another study has suggested that tumor exosomes can selectively impair lymphocyte responses to interleukin-2 Citation[20]. On the basis of these data, we re-evaluated the role of gastric cancer exosomes in immune evasion. The present study showed that gastric cancer exosomes induced apoptosis of Jurkat T cells. Furthermore, we found that activation of caspases 3, 8 and 9 was involved in exosome-induced apoptosis, which indicates that exosomes might induce Jurkat T cell apoptosis by death-receptor (caspases 3 and 8) and mitochondrial (caspases 3 and 9) pathways.
Proteasome degradation of ubiquitin-targeted proteins is an important mechanism that negatively controls activated signaling pathways Citation[21]. Cbl-b and c-Cbl are perhaps the best-studied E3 ubiquitin ligases and key regulators of T-cell signaling Citation[12], Citation[13]. The function of Cbl-b appears to be crucial for the maintenance of peripheral tolerance because Cbl-b–/– mice develop spontaneous autoimmunity Citation[13], Citation[22]. The present results indicated that the expression of proteins and mRNA of Cbl-b and c-Cbl was increased in a time-dependent manner during exosome-induced apoptosis. A recent study has reported that absence of Cbl-b results in resistance to activation-induced apoptosis in Th1 cells following CD3 ligation Citation[11]. Together, these data indicated that the Cbl family of ubiquitin ligases might play a role in the regulation of exosome-induced T-cell apoptosis.
Like many E3 ligases, Cbl-b and c-Cbl appear to ubiquitinate numerous target proteins. Fang et al. Citation[14] have shown that Cbl-b bound to and ubiquitinated p85 subunit of PI3K, and inhibited CD28-triggered PI3K/Akt activation. Moreover, Jones et al. Citation[17] have reported that CD28-dependent activation of Akt blocks Fas-mediated apoptosis and Akt transgenic T cells reduce activation of caspases 3 and 8. The present study showed that the exosome-induced up-regulation of Cbl-b and c-Cbl was accompanied by p85 subunit polyubiquitination, a decrease in activity of Akt, and activation of caspases 3, 8 and 9. Evidence from several studies has demonstrated that p85 binding Cbl family of ubiquitin ligases is highly susceptible to the ubiquitin proteasome-mediated proteolysis Citation[14], Citation[23]. The present study found that proteasome inhibitors partially restored Akt activity and reversed cell apoptosis. These results indicate that inactivation of PI3K/Akt signaling was involved in exosome-induced apoptosis, which might be dependent on the up-regulated expression of the Cbl family of ubiquitin ligases. Taken together, these observations suggest that Cbl proteins might ubiquitinate PI3K and direct proteasome-mediated degradation, thereby inhibiting Akt activity and contributing to activation of caspases 3, 8 and 9, a mechanism that might regulate exosome-induced apoptosis of T cells.
In summary, the present study reported that gastric cancer cells exploited exosome release for delivering apoptotic signals to anti-tumor T cells, without the need for direct cell-to-cell contact. This exosome effect was partially mediated by the inducible up-regulation of Cbl-b and c-Cbl, which led to inhibition of Akt activity and activation of caspases 3, 8 and 9. Furthermore, a better understanding of the molecular mechanisms involved in the interaction between exosomes and immune cells is likely to provide significant insights into the mechanism of tumor immune suppression.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.30770993, No. 30700807). Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: Molecular mechanisms and functional significance. Adv Immunol 2000; 74: 181–273
- Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: Role in immune escape. Gastroenterology 2005; 128: 1796–804
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis 2005; 35: 169–73
- Taylor DD, Gerçel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 2005; 92: 305–11
- Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–79
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: Microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94: 3791–9
- Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. “Tolerosomes” are produced by intestinal epithelial cells. Eur J Immunol 2001; 31: 2892–900
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ 2008; 15: 80–8
- Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002; 360: 295–305
- Wolfers J, Lozier A, Raposo G, Regnault A, Théry C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7: 297–303
- Hanlon A, Jang S, Salgame P. Cbl-b differentially regulates activation-induced apoptosis in T helper 1 and T helper 2 cells. Immunology 2005; 116: 507–12
- Rangachari M, Penninger JM. Negative regulation of T cell receptor signals. Curr Opin Pharmacol 2004; 4: 415–22
- Lin AE, Mak TW. The role of E3 ligases in autoimmunity and the regulation of autoreactive T cells. Curr Opin Immunol 2007; 19: 665–73
- Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3-kinase for ubiquitination in T cells. J Biol Chem 2001; 276: 4872–8
- Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 1998; 10: 262–7
- Su CC, Lin YP, Cheng YJ, Huang JY, Chuang WJ, Shan YS, et al. Phosphatidylinositol 3-kinase/Akt activation by integrin-tumor matrix interaction suppresses Fas-mediated apoptosis in T cells. J Immunol 2007; 179: 4589–97
- Jones RG, Elford AR, Parsons MJ, Wu L, Krawczyk CM, Yeh WC, et al. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J Exp Med 2002; 196: 335–48
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002; 270: 211–26
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat Med 1998; 4: 594–600
- Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res 2007; 67: 7458–66
- Roos-Mattjus P, Sistonen L. The ubiquitin-proteasome pathway. Ann Med 2004; 36: 285–95
- Bachmaier K, Krawczyk C, Kozieradzki I, Kong YY, Sasaki T, Oliveira-dos-Santos A, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 2000; 403: 211–6
- Dufour C, Guenou H, Kaabeche K, Bouvard D, Sanjay A, Marie PJ. FGFR2-Cbl interaction in lipid rafts triggers attenuation of PI3K/Akt signaling and osteoblast survival. Bone 2008; 42: 1032–9