Abstract
Background. The influence of physical activity on the development of arm lymphedema (ALE) after breast cancer surgery with axillary node dissection has been debated. We evaluated the development of ALE in two different rehabilitation programs: a no activity restrictions (NAR) in daily living combined with a moderate resistance exercise program and an activity restrictions (AR) program combined with a usual care program. The risk factors associated with the development of ALE 2 years after surgery were also evaluated. Material and methods. Women (n = 204) with a mean age of 55±10 years who had axillary node dissection were randomized into two different rehabilitation programs that lasted for 6 months: NAR (n = 104) or AR (n = 100). The primary outcomes were the difference in arm volume between the affected and control arms (Voldiff, in ml) and the development of ALE. Baseline (before surgery) and follow-up tests were performed 3 months, 6 months, and 2 years after surgery. Data were analyzed using ANCOVA and regression analysis. Results. Voldiff did not differ significantly between the two treatment groups. Arm volume increased significantly over time in both the affected and the control arms. The development of ALE from baseline to 2 years increased significantly in both groups (p < 0.001). The only risk factor for ALE was BMI > 25 kg/m2. Conclusion. Patients that undergo breast cancer surgery with axillary lymph node dissection should be encouraged to maintain physical activity in their daily lives without restrictions and without fear of developing ALE.
The development of arm lymphedema (ALE) after breast cancer surgery with axillary node dissection occurs in 6–49% of patients and its prevalence is reported to increase in the years after surgery Citation[1]. ALE can become a chronic and lifelong condition characterized by swelling and recurring skin infections Citation[2]. The characteristic swelling of tissues arises as a consequence of insufficient lymph transport caused by the surgery Citation[2]. The reported prevalence of ALE after axillary node dissection differs according to the measurement techniques used, the definition of ALE, and the follow-up time after surgery. Preoperative and valid measurements are essential to determine precisely the changes in arm volume caused by the development of lymphedema. Furthermore, the preoperative asymmetry between dominant and non-dominant limbs and the calculated percentage of increase in volume of the affected limb compared with the control limb are also essential for the determination of the exact prevalence of ALE Citation[3–5].
The reported identified risk factors for the development of ALE are radiotherapy, the number of lymph nodes removed, mastectomy, surgery on the dominant or non-dominant side, elevated body mass index (BMI), injuries, and infections Citation[1], Citation[4], Citation[6–8]. No study has examined pain and a sensation of heaviness during physical activity of the affected limb as a risk factor for the development of ALE.
Breast cancer treatment may lead to chronic pain, weight gain, and a decrease in muscle strength, cardiorespiratory fitness, shoulder function, and health-related quality of life Citation[9]. A sedentary lifestyle is a risk factor for both primary and recurrent breast cancer; however, women who undergo breast cancer surgery tend to decrease their physical activity level Citation[9], Citation[10]. The influence of postoperative exercise and the restriction of activity of the affected limb on the development of ALE after breast cancer surgery have been debated for years Citation[9], Citation[11]. Despite the evidence of positive health effects of physical activity and the positive results from studies addressing aerobic and resistance exercise training for this patient group Citation[9], Citation[12–18], physical therapists, oncologists, and nurses who participate in the rehabilitation of these patients continue to advocate restrictions in physical activity involving the affected limb as a precaution against the development of ALE. However, no increased risk of lymphedema has been found in three randomized controlled studies that assessed the effect of exercise including the upper limbs after early-stage breast cancer surgery Citation[13], Citation[14], Citation[19]. These studies included axillary node dissection and had lymphedema development (arm volume increase in ml or cm) as main outcomes Citation[13], Citation[14], Citation[19]. Thus, there is an inconsistency in the information given to patients after surgery; they are encouraged to be physically active, but, at the same time, they are cautioned to avoid several physical activities (in their daily lives and in leisure time) that would involve the upper limbs. Pain and a sensation of heaviness in the affected limb during activity have been related to the development of ALE and have been used among clinicians as an argument in support of the restriction of physical activity in these patients. Pain is a common morbidity after breast cancer treatment Citation[20–22]. A prevalence of pain up to 52% has been reported in breast cancer survivors 9 years after surgery Citation[20]. However, pain during physical activity involving the affected limb and its correlation with the possible development of ALE has not been well documented and warrants further investigation.
To our knowledge, no randomized controlled trial has studied the development of ALE, pain, and a sensation of heaviness in the affected limb after two different postoperative rehabilitation programs that involve different physical activity levels of the upper limbs. This was the main purpose of this study. Additional aims included the identification of risk factors for the development of ALE 2 years after surgery. We hypothesized that there would be no differences in arm volume or ALE between 1) patients who participated in a physical activity program with no activity restrictions (NAR), which included moderate resistance exercise training, and 2) patients who participated in a program involving physical activity restrictions (AR) of the affected limb and usual physical therapy treatment. We also hypothesized that there would be no differences in pain, or a sensation of heaviness in the affected limb between the two groups.
Material and methods
Participants
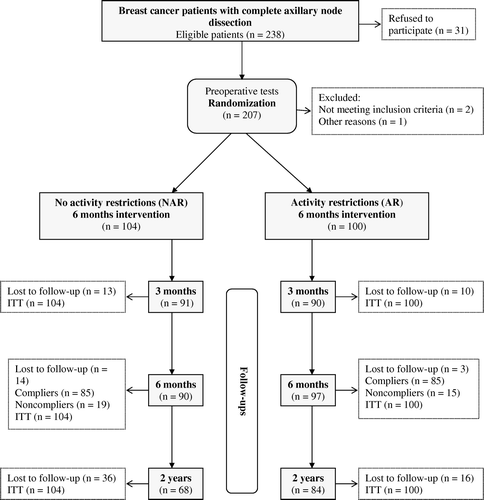
Two hundred and four women aged 32–75 (mean 55±10) years who had early-stage breast cancer and underwent mastectomy or breast-conserving surgery with axillary node dissection (levels I and II), with or without radiotherapy, chemotherapy, or hormone treatment (, ), at the Ullevaal and Akershus University Hospitals, Norway, between 1999 and 2003, were included in this study. The exclusion criteria were age > 75 years (i.e., the patients were too frail to participate in the rehabilitation programs), difficulty understanding Norwegian, and the presence of metastasized breast cancer, other types of cancer, injury, or poor functioning of the upper limb, which prevented the patients from participating in the rehabilitation programs at the outpatient clinics.
Table I. Baseline values in the no activity restrictions (NAR) and activity restrictions (AR) groups.
Protocol, assignment, and masking
This study was a randomized controlled trial with two different physical therapy interventions that lasted for 6 months. The trial had four time test points (baseline, 3 months, 6 months, and 2 years after surgery) (). Baseline measures were performed and written consent forms were obtained before surgery. Simple randomization in blocks of 10 was performed using a computer-generated program. Allocated women were randomized into one of the two intervention groups 2 days after surgery. The assignment scheme was given to the patients in sealed envelopes in a series of consecutive numbers. The allocation was masked to the outcome assessor (ÅS). It is difficult to blind the participants to allocation in physical activity studies. We took steps to blinding as we entered and managed all the data in an anonymized format. The administrative and patient contact data were held in a separate database. Information and discussions regarding the program were carried out at the outpatient clinic in collaboration with the physical therapists who supervised the rehabilitation programs. The blinded outcome assessor was not involved in the interventions performed at the outpatient clinics. Participants were instructed not to reveal information about their rehabilitation program to the outcome assessor. The regional Committee for Medical Research Ethics approved this study.
Intervention groups
After entering the trial, each patient in the NAR group was given standard detailed information on the unrestricted program in a sealed envelope. The NAR group had no restrictions on the physical activities that used the affected limb for 6 months. The NAR patients followed a supervised physical therapy program at an outpatient clinic, which emphasized moderate progressive resistance exercise training Citation[23] 2–3 times a week. The resistance exercises (total exercise time of 45 min) included a minimum of 15 repetitions for each exercise using low resistance (0.5 kg) during the first 2 weeks. The resistance was increased individually for each patient (no upper limit) with the aim of enhancing muscular strength and endurance, but always using 15 repetitions per set for each exercise.
After entering the trial, each patient in the AR group was given standard detailed information on the restricted activities in a sealed envelope. The AR group was told to restrict the activity of the affected limb for 6 months. The patients were told to avoid heavy or strenuous physical activities, which included aerobic or other types of exercise classes that include heavy upper-limb physical activity or work, and to avoid carrying or lifting groceries or other items weighing more than 3 kg. The patients also participated in the usual care physical therapy program carried out weekly at an outpatient clinic, which comprised six different standardized passive manual techniques emphasizing flexibility and light massage of the affected shoulder, arm, and scar (total intervention time of 45 min). This usual care program was performed once a week for 6 months.
Patients who developed ALE were given standard care treatment by a physical therapist specialized in lymphedema. The participants were encouraged to contact the primary investigator for evaluation and treatment of possible ALE between the follow-up sessions.
Outcome measurements
The main outcome measurement was Voldiff (in ml), which was based on the difference between the volume of the affected arm and the volume of the control arm using the Simplified Water Displacement Instrument (SWDI) Citation[3]. Two different definitions of ALE were used in this study. For the identification of risk factors for the development of ALE, a cut-off was set at Voldiff > 200 ml Citation[13]. For the determination of the incidence of ALE, a 10% increase in Voldiff between the affected arm and the control arm was used and was calculated as follows: (affected arm volume-control arm volume)/(control arm volume) 100 Citation[24].
Visual analogue scales (VAS) were used to record pain and the sensation of heaviness in the affected limb during physical activity Citation[25]. The VAS score for pain was divided into three groups: no pain (0), pain between 1–20 mm, and pain above 21 mm.
The height and weight of patients were recorded and their BMI was calculated as kg/m2.
Adherence to the intervention programs
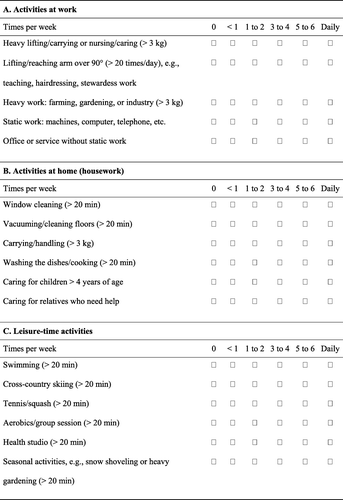
A questionnaire was developed to record upper-limb physical activity, which included the intensity, duration, and frequency of activities Citation[23]. Physical activity was categorized into three types (a,b,c): physical activity at work, at home (housework), or during leisure time. Physical activity was scored from 0 (never) to 5 (daily), with a maximum score of 50 for work activity, 30 for home physical activity, and 45 for leisure-time physical activities. The leisure-time activities were analyzed using a cut-off score level of ≥ 1 point (c). Because of the heavy postsurgical burden of radiation and chemotherapy, adherence to the rehabilitation programs was set at 70% and was defined as the number of visits to the outpatient clinics and the number of patients who completed the 2-week physical activity questionnaires ().
Statistical power and analyses
Power analysis was based on the mean Voldiff of 79 ml (standard deviation (SD) = 124), which was defined in a previous study of arm volume in the general female population using the SWDI instrument Citation[3]. We determined that 65 patients were required in each group in order to detect a minimal clinically relevant Voldiff of 50 ml between the rehabilitation groups at a two-tailed significance level < 0.05 and at 0.90 power. Two hundred and seven patients were included to account for possible missing individuals (lost to follow-up) and possible non-compliers during the intervention period ().
Outcome data were analyzed on an intention to treat (ITT) basis using the last observation carry-forward method and including all randomized patients, non-compliers, and participants lost to follow-up. Analysis of variance with baseline measurement as a covariate (ANCOVA) was used to compare differences between groups at the 3-month, 6-month, and 2-year follow-up. Bonferroni adjustments were made to allow paired comparisons for multiple testing. The Tukey's post hoc test was used to identify differences if significant main effects were found. The outcomes were expressed as means and SD, p-values (significance level of 0.05), and 95% confidence intervals (CI) were reported. The possible impact of missing values on the ITT analysis at the 2-year follow-up was tested as follows: the differences in characteristics between the study population and drop outs at 2 years were analyzed using the Student's t-test, the Mann-Whitney test, and the χ2 test. Risk factors for the development of ALE at 2 years were analyzed using multivariate logistic regression. Thirty-five participants had a Voldiff > 200 ml, which allowed the inclusion of four independent factors (10%) in the analysis. The factors chosen were BMI > 25 at baseline (before surgery), Voldiff > 0 ml at baseline, pain > 0 mm at 3 months, and a sensation of heaviness > 0 mm at 3 months. The factors were chosen based on previously reported possible risk factors for the development of ALE.
Data were analyzed using the SPSS 11.0 software (SPSS Inc, Chicago, IL).
Results
Thirty-one of the 238 eligible participants who met the inclusion criteria refused to participate. The reasons for refusal were not obtained. Two hundred and seven patients were randomized into the study. Two patients were excluded because they did not have axillary node dissection and one patient was excluded because her baseline measurements accidentally disappeared from the data collection. Finally, 204 patients were included in the statistical analyses (). The groups were balanced at baseline () and had not changed at the 2-year follow-up. Working status (e.g. whether the individual was retired, a housewife, or working at a job involving heavy lifting, which included nursing or caring for children or the elderly) did not differ significantly between the groups. The reasons for the 52 missing individuals at the 2-year follow-up examination were: 14 had died, three had moved elsewhere, 13 were not available at the given address or telephone number, seven refused to participate, four were too frail or ill, two had gone through axillary node dissection on the control side, and nine were lost during follow-up for other reasons.
Arm volume and ALE
The arm volume of the affected or control arms, Voldiff, and ALE did not differ significantly between the two groups at 3 months, 6 months, or 2 years after surgery (, ). Within each group, arm volume and ALE increased significantly with time from 3 months to 6 months and to 2 years after surgery (p < 0.001) (, ). The proportion of patients with ALE increased from 5% in the NAR group and 7% in the AR group at 3 months to 13% at 2 years for both groups.
Figure 3. Affected arm volume in ml from baseline to 2 years for the no activity restrictions (NAR) and activity restrictions (AR) groups.
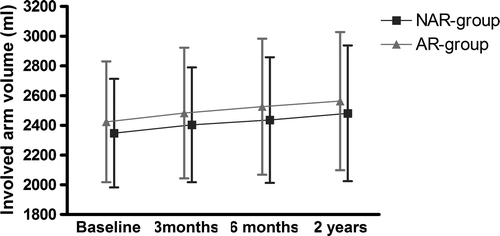
Table II. Intention to treat analysis between the (NAR) no activity restrictions (n = 104) and the (AR) activity restrictions (n = 100) groups.
Pain and sensation of heaviness
The VAS ratings of pain and sensation of heaviness for the affected limb during physical activity were significantly higher in the NAR group than in the AR group, both at 3 and 6 months after surgery ( and ). Pain and the sensation of heaviness did not differ significantly between the two groups at the 2-year follow-up ( and ). The percentages of subjects with no pain, pain between 1 and 20 mm, and pain above 21 mm on the VAS are listed in .
Table III. Pain in the affected arm during physical activity for the no activity restrictions (NAR) and activity restrictions (AR) groups.
Risk factors for the development of ALE
Individuals with a baseline BMI > 25 kg/m2 had a significantly increased risk of development of ALE at 2 years, with an Odds Ratio (OR) of 3.42 (p < 0.005) ().
Table IV. Risk factors for the development of arm lymphedema (ALE) 2 years after surgery.
Adherence to the intervention programs
The analysis of the physical activity of the upper limbs was based on home activity (housework) and leisure-time activity. Because nearly 80% of the women were not working at the 6-month follow-up, activities at work were not included (b,c). The home physical activity score was significantly higher during the time of intervention (3 and 6 months) in the NAR group than in the AR group (p < 0.001), as the NAR group had been told to not limit their level of physical activity. The physical activity scores did not differ between the groups preoperatively or at 2 years. Adherence to the allocated rehabilitation programs was 83% in the NAR group and 89% in the AR group. The mean duration of the rehabilitation programs was 21±4.8 weeks for the NAR group and 22±5.2 weeks for the AR group.
Adverse events
During the intervention programs, two participants developed adhesive capsulitis (frozen shoulder) with progressive immobilization and one patient developed supraspinatus tendinopathy. One of these women probably had a latent frozen shoulder before entering the study.
Discussion
Principal findings
This is the first randomized study to examine changes in arm volume and the development of ALE in 1) a program with unrestricted physical activity of the affected limb, which included moderate resistance exercise training, compared with 2) a restricted program for the affected limb combined with usual care. Unrestricted physical activity and moderate resistance exercises did not seem to alter the risk of developing ALE, which suggests that patients who have undergone breast cancer surgery with axillary node dissection do not need to limit the activity of the affected limb in fear of developing ALE.
Physical activity (i.e., aerobic and resistance exercise training) reduces the complications and side effects of surgical and adjuvant therapies in breast cancer survivors, which include functional limitation and upper-limb disability Citation[13], Citation[16–18]. In accordance with our results, Round et al. Citation[26] found that individuals with the best outcome were those who followed minimal activity restrictions for the affected limb and used their affected limb as much as the contralateral limb. A study of the effect of physical activity on lymphatic transport showed that higher exercise intensity involving the upper limb is more effective than low exercise intensity in promoting lymphatic clearance in healthy women. In our study, the resistance training was based on low weights and many repetitions (minimum of 15 repetitions). However, similar results were found in the study by Courneya et al., in which heavier weights and fewer repetitions were used Citation[13]. These authors also used a cut-off for ALE of > 200 ml increase in affected arm volume. Furthermore, two other randomized controlled trials (using arm circumference measurements (in cm) for the determination of ALE) found no significant differences in the incidence of ALE between the exercise and control groups Citation[14], Citation[19].
The incidence of ALE in our study increased over time in both groups, from 4% in the NAR group and 7% in the AR group at 3 months to 13% for both groups at 2 years. Similarly, Johansson et al. Citation[6] reported that the incidence of ALE increased over time in a 2-year follow-up study. At a median of 5 years of follow-up, Mclaughlin et al. Citation[4] found a 16% incidence of ALE (n = 336). However, this study used arm circumference in cm as a measure of ALE Citation[4]. The low ALE incidence detected in our study (13%) for both groups at the 2-year follow-up, when compared with other studies, may reflect the fact that (1) ALE treatment was given whenever necessary during the 6-month intervention, or when requested between the 6-month and 2-year follow-up sessions, (2) we used a more valid outcome measurement for ALE, which also included preoperative measurements Citation[5], (3) other prospective studies did not include preoperative measurements Citation[14], Citation[19], or (4) other studies has a retrospective design Citation[1], Citation[4]. The use of the 2 cm increase as a cut-off for ALE Citation[4] is inaccurate, for two reasons: first, it is not valid when compared to the gold standard water displacement technique Citation[3]; second, it does not provide a calculation of the percentage of volume increase, as a 2 cm variation in large limbs is a very small difference and, conversely, it is a very big difference in small limbs Citation[3], Citation[27].
The VAS pain ratings and the sensation of heaviness in the affected limb during physical activity were significantly higher in the NAR group than in the AR group (p < 0.001) ( and ), which had a significantly lower physical activity level (p < 0.001). However, the higher ratings of pain and the sensation of heaviness during physical activity were not correlated with Voldiff or ALE (). These results are consistent with the findings of other studies, which reported increased sensations, sensory abnormalities, and pain for this patient group Citation[20–22], Citation[25]. The intensity of arm pain during physical activity decreased over time (). More than 60% had no pain 2 years after surgery and only 16% had VAS pain scores of more than 21 mm (). The previously reported high prevalence of pain in breast cancer survivors could be one of the factors responsible for the reported decline in physical activity level after breast cancer surgery Citation[9], Citation[10]. The fact that pain and the sensation of heaviness did not seem to be risk factors for the development of ALE () is an important clinical observation. Furthermore, the higher level of pain and sensation of heaviness in the NAR group when compared to the AR group was temporary, as the groups showed identical results for these parameters 2 years after surgery ( and ).
The score of physical activity at home during the rehabilitation period, which was assessed every two-weeks, was significantly higher in the NAR group than in the AR group. These results showed that the NAR group followed the allocated intervention program and used their affected limb significantly more than the AR group. The leisure-time activity scores were low for both groups and did not differ between the groups. Previous studies also reported low physical activity levels for breast cancer patients Citation[9], although this level increases when household and gardening activities are included Citation[10].
As previously reported by others Citation[1], Citation[4], Citation[6], Citation[7], we found that a BMI > 25 kg/m2 was a significant risk factor for the development of ALE. A preoperative BMI > 25 kg/m2 represented a more than three times greater risk of developing ALE at 2 years (). The implications of these results for clinical practice should be to implement patient education programs that include evidence-based recommendations for physical activity for patients who have undergone breast cancer surgery.
The basic mechanisms underlying the development of lymphedema as a result of breast cancer surgical interventions are probably multifactorial Citation[2]. There are genetic features that predispose to vascular malfunction or insufficient repair after trauma, which includes surgery Citation[2], Citation[28]. Improved knowledge on this inherited predisposition is probably the key that will allow the prediction of the development of ALE after breast cancer surgery. However, increased BMI was a significant, modifiable, and clinically important risk factor for the development of ALE.
Strengths and weaknesses of the study
The main limitation of this study was the number of patients lost at the 2-year follow-up (36 in the NAR group and 16 in the AR group). However, the baseline characteristics did not differ significantly between patients who completed the study and those missing at follow-up and per protocol analysis showed the same results as the ITT analysis. Furthermore, our a priori statistical power analysis showed that, at 2 years, there was still enough statistical power to detect potential differences (if present). Other limitations included the physical activity questionnaire for the upper limbs, which has not been validated (). Our study population had the advantage of being representative of the breast cancer community, as 32% of our patients were in the age group older than 60 years, i.e., close to the reported percentage of breast cancer in that group Citation[29]. Previous studies on physical activity and the development of lymphedema in breast cancer patients included younger participants Citation[13], Citation[14], Citation[19]. Additional strengths of the current study were the reliable and valid measurement of ALE, the long follow-up period of 2 years, the detailed description of the interventions, the high adherence to the intervention programs (83% in the NAR group and 89% in the AR group), the activity recordings during the entire 6-month period, and the loss of few participants during the intervention period (14 in the NAR group and three in the AR group) ().
Conclusions and implications for clinical practice
No differences in arm volume, Voldiff, or ALE were found between the groups. Increased physical activity was temporarily painful for the first 6 months after surgery, but pain scores were equal between the two groups after 2 years. In contrast, we found no adverse effects of unrestricted upper-limb activity on arm volume, Voldiff, or the development of ALE. A BMI > 25 was a risk factor for the development of ALE.
Our study suggests that patients that undergo breast cancer surgery with axillary node dissection should be encouraged to maintain their daily living activities, with no restrictions to the physical activity level of the affected limb and without fear of developing ALE.
Acknowledgements
We are most grateful to all the women who participated in the study. We wish to thank professor Leiv Sandvik for statistical assistance. We also thank physical therapist Hans Petter Faugli for helpful assistance with the performance of the resistance training program, the physical therapists who supervised the patients during the intervention programs, and Brit Magnell and Jørn Rosenberg for practical administration, patient assessment, and lymphedema treatment.
This study was financially supported by Health and Rehabilitation, the Norwegian Cancer Society, and The Norwegian Women's Public Health Association.
References
- Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92: 1368–77
- Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol 2008; 52: 799–806
- Sagen A, Karesen R, Risberg MA. The reliability of a simplified water displacement instrument: A method for measuring arm volume. Arch Phys Med Rehabil 2005; 86: 86–9
- McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol 2008; 26: 5213–9
- Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008; 112: 2809–19
- Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. Factors associated with the development of arm lymphedema following breast cancer treatment: A match pair case-control study. Lymphology 2002; 35: 59–71
- Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: A three-year follow-up study. QJM 2005; 98: 343–8
- Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J Clin Oncol 2008; 26: 3536–42
- Doyle C, Kushi LH, Byers T, Courneya KS, Mark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin 2006; 56: 323–53
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol 2008; 26: 3958–64
- Rockson SG. Precipitating factors in lymphedema: Myths and realities. Cancer 1998; 83: 2814–20
- McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. CMAJ 2006; 175: 34–41
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007; 25: 4396–404
- Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol 2006; 24: 2765–72
- Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. BMJ 2007; 334: 517
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol 2007; 25: 1713–21
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: Results of a randomized controlled trial. J Clin Oncol 2001; 19: 657–65
- Milne HM, Wallman KE, Gordon S, Courneya KS. Impact of a combined resistance and aerobic exercise program on motivational variables in breast cancer survivors: A randomized controlled trial. Ann Behav Med 2008; 36: 158–66
- Basen-Engquist K, Taylor CL, Rosenblum C, Smith MA, Shinn EH, Greisinger A, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns 2006; 64: 225–34
- Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 2005; 92: 225–30
- Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: A prospective study. J Pain 2006; 7: 626–34
- Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: An investigation of women's experiences. Pain 1995; 61: 61–8
- American College of Sports Medicine. ACM‘s guidelines for exercise testing and prescription. 7th ed. Lippincott Williams & Wilkins; 2006.
- Swedborg I. Voluminometric estimation of the degree of lymphedema and its therapy by pneumatic compression. Scand J Rehabil Med 1977; 9: 131–5
- Gottrup H, Andersen J, Rendt-Nielsen L, Jensen TS. Psychophysical examination in patients with post-mastectomy pain. Pain 2000; 87: 275–84
- Round T, Hayes SC, Newman B. How do recovery advice and behavioural characteristics influence upper-body function and quality of life among women 6 months after breast cancer diagnosis?. Support Care Cancer 2006; 14: 22–9
- Gebruers N, Truijen S, Engelborghs S, De Deyn PP. Volumetric evaluation of upper extremities in 250 healthy persons. Clin Physiol Funct Imaging 2007; 27: 17–22
- Rockson SG. Secondary lymphedema: Is it a primary disease?. Lymphat Res Biol 2008; 6: 63–4
- Bhatia S, Robison LL. Cancer survivorship research: Opportunities and future needs for expanding the research base. Cancer Epidemiol Biomarkers Prev 2008; 17: 1551–7