Abstract
Background and purpose. Many breast cancer survivors (BCS) suffer from long-term upper limb morbidities after axillary node dissection. The purpose of this five-year follow-up study was to describe changes in long-term upper limb morbidities, physical activity level, and Health-Related Quality of Life (HRQoL) and to find factors that predict HRQoL five years after surgery. Patients and methods. This study included 204 women aged 55±10 years who had primary breast cancer surgery with axillary node dissection. The subjects were examined for arm volumes and arm lymphedema, arm pain, sensation of heaviness, shoulder function, physical activity level, and HRQoL, prior to surgery, and six months and five years after surgery. The statistical analyses used included ANOVA for repeated measures and multivariate linear regression. Results. ALE (13%), pain (36%), and sensation of heaviness (21%) in the upper limbs were present five years after surgery. ALE was the only morbidity that continued to increase over time. Several dimensions of HRQoL temporarily declined after surgery, but significantly improved in the period from six months to five years after surgery. The significant predictive factors of HRQoL five years after surgery included HRQoL prior to surgery, physical activity level at leisure time (both prior to and at six months after surgery), and duration of sick leave after surgery (in weeks). Conclusions. The overall HRQoL improved significantly from baseline to five years, despite the chronic arm pain and increase in ALE. Three independent predictive factors of HRQoL were identified.
The majority of breast cancer survivors (BCS) have long-term upper limb and shoulder morbidities, including lymphedema, pain, and reduced shoulder function, and have a lower physical activity level compared with activity level prior to surgery Citation[1]. Moreover, increased physical activity has been shown to reduce these morbidities, as well as the incidence of relapse for breast cancer, and to increase Health Related Quality of Life (HRQoL) Citation[1–4]; however, long-term follow-up studies on upper limb morbidities and HRQoL in BCS after axillary node dissection (ALND) are sparse Citation[4–7].
Arm lymphedema (ALE), is commonly associated with breast cancer surgery with ALND and is most frequently reported to be influenced by the number of nodes removed and high body mass index (BMI) Citation[8–12]. The incidence of ALE increases over time and varies according to differences in follow-up time, definition of and methods used for measuring ALE, and study design Citation[13], Citation[14]. Preoperative assessments of arm volume have led to a more precise prevalence of ALE Citation[13]; however, we only identified one study including measurement of preoperative ALE and long-term follow-up (prevalence of ALE =16%) Citation[9]. Other long-term arm morbidities, such as chronic arm pain, have a prevalence of 20–50% five to 12 years after surgery Citation[15], Citation[16] and are strongly associated with HRQoL Citation[16].
The HRQoL outcomes for patients with locally advanced breast cancer have reported to decrease temporarily during cancer treatment, but return to baseline levels after treatment Citation[17]. The cancer treatment seems to affect several parameters included in HRQoL questionnaires Citation[3], Citation[4]. Women diagnosed with ALE, arm pain or other arm symptoms have shown to have a lower physical and mental HRQoL compared to BCS without ALE or arm symptoms Citation[6], Citation[18]. In addition, physical activity level and chemotherapy are associated with changes in physical functioning Citation[3]; however, cancer treatment does not seem to influence negatively the overall long-term HRQoL in BCS Citation[4].
The primary aim of this prospective five-year cohort study was to describe changes in arm volume and ALE, arm and shoulder symptoms, physical activity level and HRQoL, prior to surgery and six months and five years after surgery. Our secondary aim was to identify predictive factors for the HRQoL five years after breast cancer surgery.
Material and methods
Patients
This study included 204 women with an average age of 55±10 years (32 to 75 years) with early stage breast cancer (stages I and II) and who underwent mastectomy or breast-conserving surgery with ALND (levels I and II), with or without radiotherapy, chemotherapy, or hormone treatment (). Patients were recruited before surgery between 1999 and 2003 at the Ullevaal and Akershus University Hospitals in Norway. Subjects participated in a randomized controlled intervention study with two different physical therapy regimens during the first six months that showed no significant differences in outcome between the two rehabilitation groups Citation[12]. The exclusion criteria were: patients older than 75 years of age, too fragile to participate, problems understanding Norwegian, metastasized breast cancer, other cancer diseases, and injuries that affected the functioning of the upper limbs. The participants were given physical therapy and lymphedema treatment (when needed) during the five-year follow-up period.
Table I. Demographics, preoperatively and at the five-year follow-up time point.
Measurements
The participants were assessed at baseline (prior to surgery) and prospectively at six months and five years after surgery.
Arm volume (milliliters) and difference in arm volume between the affected and control arms (Voldiff) were measured using the Simplified Water Displacement Instrument (SWDI) Citation[19]. Definition of ALE was based on a criteria commonly used in the literature: a 10% increase in the affected arm volume compared with the control arm volume ((affected – control)/control)×100) Citation[20].
Pain and sensation of heaviness in the affected limb during physical activity and at rest were evaluated using visual analogue scales (VAS) Citation[20] and were assessed by the participants assisted by a physical therapist. Sensation of heaviness in the affected limb is part of the variety of sensations in the post mastectomy pain syndrome Citation[21] and has previously been used as an outcome measurement for lymphedema patients Citation[6], Citation[7].
Shoulder function was evaluated using the “shoulder, elbow, and wrist movement impairment” score, validated and reliability tested for women with rheumatoid arthritis Citation[22].
Height and weight were recorded to determine BMI (kg/m2).
Physical activity during leisure times was assessed using a questionnaire that included the frequency per week of sessions of moderate to vigorous physical activity lasting at least 20 min. The questionnaire has previously been used to asses adherence to physical activity interventions Citation[12].
The HRQoL was evaluated using The Core Quality of life Questionnaire of the European Organization for Research and Treatment of Cancer (EORTC QLQ-C30, version 3.0) Citation[23]. The raw scores were calculated into scales (0–100) using the recommended EORTC procedures. The 30 questions were sectioned into five scales: physical functioning, functioning, symptoms, fatigue, and global health status.
Statistical analyses
The planned sample size of 204 participants was based on power calculations of the primary end point (ALE) Citation[12]. Analysis of Variance (ANOVA) for repeated measures was used to assess the differences in arm volume, BMI, physical activity level, shoulder function, and HRQoL scales between baseline, six-month, and five-year follow-ups. Student's t-tests were used to assess pain and sensation of heaviness in the affected limb at six months and five years after surgery (no preoperative data for pain and sensation of heaviness were recorded). Bonferroni adjustments were made to allow paired comparisons for multiple testing. We included descriptive data, 95% confidence intervals (CI), and significance tests for hypothesized changes (the significance threshold was set at p < 0.05). To identify predictive factors for the HRQoL at the five-year follow-up, the following model was used: relevant predictive factors were analyzed using a univariate linear regression analysis; in cases where p was lower than 0.05 (two-tailed), the significant factors were included into a multivariate linear regression analysis with forward variable selection. Data were analyzed using the SPSS 11.0 software (SPSS Inc, Chicago, IL).
Ethics
The regional Committee for Medical Research Ethics approved the study and the patients signed a written consent form prior to participation in the study.
Results
One hundred and fifty-seven (77%) of the 204 women originally recruited, participated at the final five-year follow-up (). Seven women had recurrence but participated in the follow-up at five years, 28 (14%) died, six moved elsewhere, five were not available via telephone or at their address, and eight refused to participate in the five-year follow-up.
Arm lymphedema was found in 12 women (7%) at six months follow-up and increased to 21 women (13%) at the five-year-follow-up (). The change in Voldiff increased significantly from baseline to six months, but not from the six-month to the five-year follow-ups (). The mean BMI increased significantly from baseline to six months, but not from baseline to five years ().
Table II. Pain and sensation of heaviness and arm lymphedema (ALE) for the affected limb.
Table III. Changes* in arm volume and BMI.
During physical activity arm pain was observed in 56% of the woman at 6-months follow-up, and at rest in 60% of the women. At the five-years follow-up the number of women with pain decreased significantly to 36% and 30%, respectively (). Sensation of heaviness was detected in 46% of the women during activity and in 45% during rest at six months, and decreased significantly to 21% and 20% at 5 years, respectively ().
Four of the five function scores for the affected shoulder significantly decreased from baseline to six months (from 29.7±0.8 points to 28.9 ±1.9 points) (p < 0.001), but there were no significant changes from baseline to five years (p = 0.171). The control side scores did not change over time.
The mean physical activity level did not change significantly (CI, −3 to 25) (p = 0.167) from baseline to six months, or from baseline to five years (CI, −17 to 20) (p = 1.000).
The HRQoL scale for Physical Functioning did not change significantly over time (, ), but the Functioning scale decreased significantly over time from baseline to five years (, ). The Symptoms scale and the Fatigue scale significantly increased temporarily from baseline to six months, but did not change significantly from baseline to five years (, ). The Global Health scale significantly increased over time from baseline to five years (, ).
Figure 1. Figure 1. Arm volumes from baseline (n=204) (before surgery) to five years (n=157) after surgery.
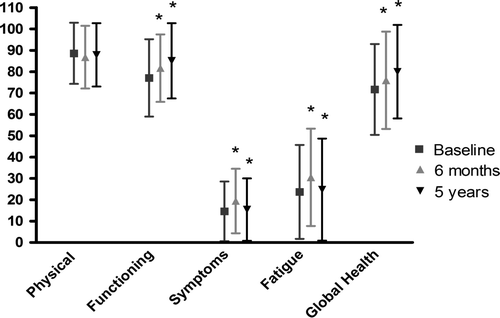
Figure 2. HRQoL scales from baseline (n = 204) (before surgery) to five years (n = 157) after surgery. *p < 0.05.
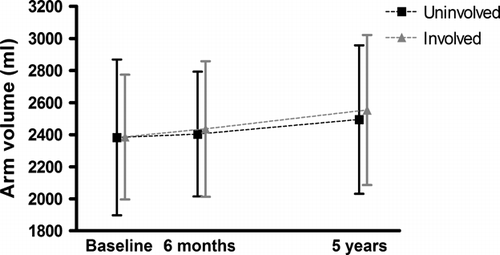
Table IV. Changes* in the HRQoL scales from baseline to 6 months and 5 years.
The multivariate analysis showed that the physical activity level at leisure time both at baseline and at six-month follow-up were a significant predictive factor of the Physical Functioning scale at five years (). The multivariate analysis revealed that the duration of sick leave at six months was the only significant predictive factor of Functioning at five years (). The multivariate analysis showed that the Symptoms scale prior to surgery was the only significant predictive factor of Symptoms at five years (). The multivariate analysis revealed that both the Global Health scale prior to surgery and the duration of sick leave at six months were significant predictive factors of Global Health status at five years ().
Table V. Predictive factors for HRQoL scales at 5 years after surgery.
Discussion
To our knowledge, this is the first five-year follow-up study that evaluated upper limb morbidities including preoperative assessment of arm volume, arm and shoulder symptoms and HRQoL in breast cancer patients with ALND.
As shown in other studies, we found that the incidence of ALE increased by time after surgery Citation[11], Citation[12]. Similarly we, as the others, found a significantly increased arm volume from baseline to six months after surgery, both in the affected and in the control arms Citation[2], Citation[13], Citation[24]. However we did not find a significant increase in arm volume from six months to five years (, ). Furthermore, BMI increased exclusively during the first six months (), which is in accordance with previous reports Citation[8], Citation[12]. Accordingly the first six months seems to be most important if one plans interventions to prevent this development.
Studies with preoperative assessments tend to report lower rates of ALE Citation[9], Citation[11], Citation[12] than studies without preoperative data on arm volume. Furthermore, studies of self-reported data for lymphedema and arm symptoms tend to report the highest incidences Citation[18]; Norman et al. reported a prevalence of 42% within 5 years after diagnosis Citation[7] and Meeske et al. reported an incidence of 24% within 18 months after the diagnosis Citation[10]. However, Ahmed et al. only disclosed lymphedema in 8% of the BCS eight years after the diagnosis by using the same self-report measure of lymphedema as Norman Citation[6]. These huge differences in the incidence of ALE by using the same instrument may indicate poor validity. Closer to our low results of ALE (13%) () was the reported incidence of 16% in a five-years follow-up by Mclaughlin et al. Citation[9] who included preoperative objective measurements.
Previous studies have found a lower incidence of ALE in patients given physical therapy treatment compared to those who did not receive such treatment Citation[13]. The incidence of ALE increased from 7 to 13% in the period between six months to five years. Our low incidence could be the result of the close follow-ups and the physical therapy (including ALE treatment) given to these patients Citation[12].
Arm pain was still present five years after surgery, both at rest (20%) and during physical activity (21%), but the numbers of women with pain had significantly decreased since six months after surgery (). We have previously reported that the intensity of arm-pain and sensation of heaviness two years after surgery was mainly low in these patients Citation[12]. Similar results have recently been reported in a five-year follow-up study in Denmark, where 29% of the BCS developed chronic pain Citation[16]. It has been suggested that BCS with chronic pain symptoms including neurological dysfunction such as sensation of heaviness, have been undertreated, and have obtained poor pain relief from their symptoms Citation[21].
The shoulder function declined significantly the first six months, but improved to the baseline level at five years. Previous studies have also reported significant shoulder dysfunction in BCS, but patients given physical therapy shortly after surgery return a state of almost normal shoulder function Citation[25]. As with ALE the first six months seems to be the best intervention period if one will prevent bad shoulder function.
The previously reported decline in physical activity level after breast cancer surgery Citation[1], Citation[3] was not found in our study. The mean physical activity level at leisure time was similar to that at baseline, 126±84 min per week, and did not decline significantly at six months or at five years after surgery (). The recommended physical activity level has been defined as 150 min per week of moderate to vigorous intensity Citation[26]. Among the 806 BCS in the American Health, Eating, Activity and Lifestyle Study, 32% had a physical activity level at the recommended 150 min per week Citation[27]. Prior to surgery, 36% of the participants in our study had a physical activity level during leisure time above the recommended level of 150 min per week. Five years later, the physical activity level in 34% of the participants was above the recommended level. Fifty-one percent of the Norwegian general female population above 60 years of age, have reported that they are physical active during leisure time (brisk walking, bicycling etc) more than two times per week Citation[28]. The reported activity level was lower than for the 34% of the BCS in our study that was above 150 min/week. The numbers of physical active BCS in our study is almost similar to the general population.
The HRQoL in our subjects was better than for the age-matched Norwegian female population Citation[23], and is in accordance with the previously reported increase in HRQoL in disease-free physical active BCS Citation[3], Citation[4]. Late morbidities after breast cancer affect several subscales of HRQoL Citation[4–6], Citation[23]. The Functioning scale decreased significantly by -8.6 points (, ), which is in accordance with previous results Citation[4–6]; however, the previously reported decrease in Physical Functioning values in BCS (for the same age group) was not found in our study Citation[4] (, ). The Global Health status increased significantly by 7.3 points, from the baseline to five years after surgery (, ), and was more than 10 points above the mean value reported of 69.9±25.8 for the general female population for the age group between 70 and 79 years of age, and five points above the general female population aged 60 to 69 years Citation[23]. As previously reported, the women who participated in this study were included based on the criteria of being able to participate in physical therapy rehabilitation with resistance exercise training at an outpatient clinic Citation[12]. Patients who were too frail were excluded. This might be a reason for the high Physical Functioning and Global health status scores in our study when compared to the general population Citation[23].
Chemotherapy was found to be a significant predictive factor for decreased Global Health status five years after surgery, as assessed by univariate analysis (). This was in accordance with the results reported by Ganz et al.Citation[29]; However, the multivariate analysis revealed that chemotherapy was not a significant predictive factor (). Treatment with chemotherapy and high demands at work were reported to be of significance for symptom persistence 10 months after surgery Citation[3].
Acute pain after surgery is a risk factor for developing chronic pain in BCS Citation[29]. This observation is corroborated by univariate analysis of our material, showing that arm pain three months after surgery is a predictive factor for increased Symptoms at five years ().
The physical activity level (at baseline and at six months after surgery), obtained by both univariate and multivariate analysis, was a significant predictive factor for the Functioning and for the Physical functioning scales five years after surgery (). The significant relationship between an increased physical activity level and increased HRQoL in BCS has been previously reported Citation[3], Citation[29] and is further supported by our findings.
The duration of the sick leave after surgery appeared to be a significant predictive factor for both Functioning and the Global Health Status (). Sick-listed and unemployed individuals in the general population have also reported lowered self-rated health and lowered social participation Citation[30].
The 47 patients (23%) who were lost to follow-up at 5 years might be a limitation in our study. However, the lost cases did not affect the power or biased the results because intentionally the number of patients included were set high enough to allow this number of drop-outs without loosing the necessary statistical power Citation[12]. Furthermore, we have presented the results of change over time based on ANOVA for repeated measures, a favorable analysis for data at different time-points along the time axe because the numbers of significant-tests are reduced to one. However, we believe that the assessment of arm pain preoperatively would have enabled to examine changes in pain score more precisely. The necessary data to provide such results was not obtained.
Arm pain (36%) and a sensation of heaviness (32%) might be reduced by systematic registration and treatment shortly after surgery Citation[12]. Our fairly low frequencies of ALE (13%) and recovery from shoulder motion impairments can probably be attributed to the physical therapy program in our study. Our data are, however, too limited to support a firm conclusion. All patients that have gone through axillary node dissection should be encouraged to a physical active life and treatment instituted as soon as ALE is detected. Future clinical practice should integrate measurements of ALE, pain and shoulder function during routine follow-up visits and adequate therapy for those with pain, beginning lymphedema and shoulder motion impairments.
Conclusions
Arm lymphedema, pain, and the sensation of heaviness in the limb were still present five years after surgery. Arm lymphedema was the only morbidity that continued to increase over time. The HRQoL temporarily declined, but later significantly improved from six months to five years. The significant predictive factors for HRQoL five years after surgery were: HRQoL prior to surgery, physical activity level at leisure time (both prior to and at six months after surgery), and duration of sick leave after surgery (in weeks).
Acknowledgements
We are most grateful to all the women who took part in the study. Norwegian Foundation for Health and Rehabilitation, the Norwegian Cancer Society, and the Norwegian Women's Public Health Association financially supported this study. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Doyle C, Kushi LH, Byers T, Courneya KS, Mark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J Clin 2006; 56: 323–53
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol 2007; 25: 4396–404
- Alfano CM, Smith AW, Irwin ML, Bowen DJ, Sorensen B, Reeve BB, et al. Physical activity, long-term symptoms, and physical health-related quality of life among breast cancer survivors: A prospective analysis. J Cancer Surviv 2007; 1: 116–28
- Peuckmann V, Ekholm O, Rasmussen NK, Moller S, Groenvold M, Christiansen P, et al. Health-related quality of life in long-term breast cancer survivors: Nationwide survey in Denmark. Breast Cancer Res Treat 2007; 104: 39–46
- Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008; 112: 2577–92
- Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: The Iowa Women's Health Study. J Clin Oncol 2008; 26: 5689–96
- Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: Incidence, degree, time course, treatment, and symptoms. J Clin Oncol 2009; 27: 390–7
- Johansson K, Ohlsson K, Ingvar C, Albertsson M, Ekdahl C. Factors associated with the development of arm lymphedema following breast cancer treatment: A match pair case-control study. Lymphology 2002; 35: 59–71
- McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: Objective measurements. J Clin Oncol 2008; 26: 5213–9
- Meeske KA, Sullivan-Halley J, Smith AW, McTiernan A, Baumgartner KB, Harlan LC, et al. Risk factors for arm lymphedema following breast cancer diagnosis in Black women and White women. Breast Cancer Res Treat 2009; 113: 383–91
- Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: A three-year follow-up study. QJM 2005; 98: 343–8
- Sagen, Å, Kaaresen, R, Risberg, MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective randomized, controlled trial with two years follow-up. Acta Oncol 2009; 48:1102–10.
- Stout Gergich NL, Pfalzer LA, McGarvey C, Springer B, Gerber LH, Soballe P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008; 112: 2809–19
- Armer JM. The problem of post-breast cancer lymphedema: Impact and measurement issues. Cancer Invest 2005; 23: 76–83
- Macdonald L, Bruce J, Scott NW, Smith WC, Chambers WA. Long-term follow-up of breast cancer survivors with post-mastectomy pain syndrome. Br J Cancer 2005; 92: 225–30
- Peuckmann, V, Ekholm, O, Rasmussen, NK, Groenvold, M, Christiansen, P, Moller, S, et al. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur J Pain 2008; July 15.
- Bottomley A, Flechtner H, Efficace F, Vanvoorden V, Coens C, Therasse P, et al. Health related quality of life outcomes in cancer clinical trials. Eur J Cancer 2005; 41: 1697–709
- Dawes DJ, Meterissian S, Goldberg M, Mayo NE. Impact of lymphoedema on arm function and health-related quality of life in women following breast cancer surgery. J Rehabil Med 2008; 40: 651–8
- Sagen Å, Kåresen R, Risberg MA. The reliability of a simplified water displacement instrument: A method for measuring arm volume. Arch Phys Med Rehabil 2005; 86: 86–9
- Hladiuk M, Huchcroft S, Temple W, Schnurr BE. Arm function after axillary dissection for breast cancer: A pilot study to provide parameter estimates. J Surg Oncol 1992; 50: 47–52
- Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: An investigation of women's experiences. Pain 1995; 61: 61–8
- Bostrom C, Harms-Ringdahl K, Nordemar R. Relationships between measurements of impairment, disability, pain, and disease activity in rheumatoid arthritis patients with shoulder problems. Scand J Rheumatol 1995; 24: 352–9
- Fosså SD, Hess SL, Dahl AA, Hjermstad MJ, Veenstra M. Stability of health-related quality of life in the Norwegian general population and impact of chronic morbidity in individuals with and without a cancer diagnosis. Acta Oncol 2007; 46: 452–61
- Hayes SC, Janda M, Cornish B, Battistutta D, Newman B. Lymphedema after breast cancer: Incidence, risk factors, and effect on upper body function. J Clin Oncol 2008; 26: 3536–42
- Rietman JS, Geertzen JH, Hoekstra HJ, Baas P, Dolsma WV, de VJ, et al. Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy for stage I or II breast cancer. Eur J Surg Oncol 2006; 32: 148–52
- U.S. Department of Health and Human Services. Physical activity and health: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services. 1996;9.
- Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: The health, eating, activity, and lifestyle study. J Clin Oncol 2008; 26: 3958–64
- Sogaard AJ, Bo K, Klungland M, Jacobsen BK. A review of Norwegian studies–how much do we exercise during our leisure time?. Tidsskr Nor Laegeforen 2000; 120: 3439–46
- Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: A follow-up study. J Natl Cancer Inst 2002; 94: 39–49
- Molarius A, Berglund K, Eriksson C, Lambe M, Nordstrom E, Eriksson HG, et al. Socioeconomic conditions, lifestyle factors, and self-rated health among men and women in Sweden. Eur J Public Health 2007; 17: 125–33