Abstract
While novel endocrine treatment options have been implemented in the advanced – as well as adjuvant setting, recent results suggest a place for “old-fashioned” additive treatment with estrogens in advanced breast cancer. This paper reviews the biological rationale for endocrine therapy in general and additive treatment with estrogens in particular. The finding that patients becoming resistant to treatment with aromatase inhibitors may subsequently respond to estrogen therapy adds important information to our understanding of therapy resistance in general. Moreover, the return of a therapeutic option abandoned more than 20 years ago, now to be used in a different sequential setting, suggests a critical examination whether there may be other conventional treatment options still earning a place as treatment in advanced disease as well. While ablative therapies including surgical oophorectomy, hypophysectomy and adrenalectomy are not candidate treatment options due to morbidity, there are additive treatment options apart from estrogen therapy that may be considered. Androgens administered at therapeutic doses are not feasible for toxicity reasons; yet, the potential of adding androgens in small doses as adjuvant to aromatase inhibitors should be further explored. Whether patients become resistant to other treatment options may still benefit from megestrol acetate, remains to be explored.
The past two decades have been a time period of continuous improvement of endocrine treatment for breast cancer. Thus, contemporary therapy includes SERMs, the SERDs, LH-RH analogues and third-generation aromatase inhibitors.
These therapeutic options have successfully replaced conventional endocrine therapy options. In general, contemporary treatment strategies have been selected based on less side effects compared to conventional treatment. While some previous strategies, including additive therapy with glucocorticoids or attempts to inhibit adrenal enzymes with drugs like ketoconazole and trilostane were abandoned due to a low anti-tumour efficacy, apart from implementation of the novel third-generation aromatase inhibitors, contemporary treatment has not improved efficacy in comparison to conventional strategies as hypophysectomy, adrenalectomy or additive treatment with estrogens.
Considering treatment of advanced breast cancer to be palliative, side effect profile represents a reasonable rationale for therapy selection. Yet, selection of one therapeutic option may not necessarily imply the second option should be completely abandoned from clinical use. Some breast cancers may respond to multiple treatment options applied in sequence Citation[1–3]; probably the greatest mistake we make with such patients is to deprive them of the full benefits of extended endocrine therapy before moving into other treatment options. For those patients, several of the more “toxic” endocrine treatment options may be associated with a significantly better quality of life as compared to chemotherapy.
Implementation of novel endocrine compounds in the adjuvant setting has challenged optimal use of treatment options for relapse. For patients experiencing a long disease-free interval after adjuvant treatment, this could mean re-implementing the same compounds as used for adjuvant therapy. However, many patients are in need of second- and third-line treatment options as well, underlining the need for more treatment options. Now, estrogen additive therapy has been successfully reintroduced in this setting Citation[3], Citation[4], revealing anti-tumour efficacy even among heavily pre-treated patients Citation[3]. This finding should trigger a critical examination of conventional endocrine treatment exploring whether other therapeutic options may serve a place in treatment of advanced disease as well. For some options, as additive treatment with progestins and estrogens as well as ablative treatment with hypophysectomy, the mechanisms behind the anti-tumour effects remains incomplete understood. Thus, before examining a potential role for traditional treatment options, the biological rationale for endocrine therapy should be explored.
The biological rationale for endocrine therapy
This section will review the rationale for estrogen manipulation, as this provides the background to most endocrine treatment. Considering the exception – additive treatment with use of androgens – the endocrine rationale will be briefly considered when discussing this treatment option.
While the identification of estrogens has been dedicated to different investigators in the 1920s, Steinach in 1937 Citation[5] was the first to confirm androgens to be converted into estrogen compounds in animals. While empirical evidence substantiated endocrine therapy for decades, the scientific rationale for endocrine therapy in breast cancer was provided by Elwood Jensen's discovery of the ER in breast tumour tissue in the 1960s Citation[6]. This was followed by subsequent studies by McGuire's team revealing its prognostic as well as predictive value with respect to endocrine therapy Citation[7], soon to be confirmed by others, including the Danish Breast Cancer Group Citation[8], Citation[9]
Role of the estrogen and progesterone receptors as predictive factors in endocrine therapy
While it has been generally accepted for decades that tumours lacking expression of the ER as well as the PgR are not sensitive to endocrine manipulation Citation[7], Citation[10], there is no general consensus with respect to the limit defining ER+. While previous assays evaluated the ER by ligand binding techniques Citation[11], currently immunohistochemistry has become the gold standard Citation[12].
In a large study Harvey et al. compared ER values determined by ligand binding assays to values obtained by immuno-expression in 2000 primary breast cancer cases. Developing an immuno-histochemical score taking into account percentage of positive staining cells as well as staining intensity Citation[12], the authors compared individual tumour score to the value obtained with the ligand binding assay Citation[13]. The result revealed immuno-expression to be superior compared to the ligand binding assay with respect to outcome. Interestingly from a biological point of view, positive expression in 1% of the cells only provided prognostic information compared to ER negativity among patients receiving tamoxifen treatment Citation[13].
We may not explain why tumours expressing positive staining for the ER in, say, 10% of the cells only, may respond to endocrine therapy. Neither do we know the mechanisms of primary as well as acquired resistance in ER + breast cancer. However, it is old wisdom that tumours expressing ER at high levels have a better chance of responding to endocrine manipulation compared to tumours expressing ER at low levels Citation[7]; also, there is evidence that co-expression of the progesterone receptor (PgR), considered a marker of ER functionality Citation[14], predicts a better likelihood of responsiveness in metastatic as well as in the adjuvant setting Citation[10], Citation[15]. As for ablative as well as additive endocrine therapies, most treatment options were in clinical use before the time of routine assessment of the ER. However, while we may not determine the predictive role of receptor expression to these therapies, there is little reason to hypothesise the predictive role of ER to be much different with respect to these conventional strategies as it is for contemporary treatment.
Estrogen disposition in women
Plasma estradiol (E2) levels vary substantially across the menstrual cycle in premenopausal women. Average levels in the range of 200–600 pmol/l may be recorded, but with pre-ovulatory peak levels reaching 1 000–2 000 pmol/L. This strongly contrasts the plasma levels observed in postmenopausal women, with an average level of less than 20 pmol/L as measured by highly sensitive methods calibrated for measurement in the low range Citation[16]. Plasma levels of the inactive estrogen estrone (E1) may average 60–80 pmol/L in postmenopausal women Citation[16] and 2–300 pmol/L in premenopausals. Inactive as a receptor ligand, breast cancer and benign tissues contain the enzymes required to convert E1, and also its conjugate E1S, into active E2Citation[17], Citation[18]. The reason for a different E2/E1 ratio among pre- and postmenopausal women is pharmacokinetic; while the ovary secretes E2, with a minor contribution of E1, postmenopausal estrogens are synthesised in peripheral tissue mainly as E1 by uptake of circulating androstenedione (see Citation[19] for details). Androgens are synthesised mainly in the adrenal glands, with a minor contribution from the postmenopausal ovary Citation[19], Citation[20]. Average levels of plasma androstenedione and testosterone are about 5 nmol/l and 1.5 nmol/L in postmenopausal women Citation[21]. As the peripheral aromatase has a preference for androstenedione compared to testosterone as substrate, this explains why E1 is the main estrogen product in postmenopausal women, with E2 being synthesised partly through aromatisation of testosterone, partly by reduction of E1Citation[19].
The finding by van Landeghem and colleagues Citation[22] that breast cancer tissue E2 may exceed plasma E2 levels in postmenopausal women, approaching intra-tumour levels recorded in premenopausal breast cancer, has lead to the belief that breast cancer estrogen levels are due to local synthesis with a minor contribution from the circulation. Such a hypothesis may not conflict observations on the effects of adrenalectomy, hypophysectomy or treatment with aromatase inhibitors; adrenal suppression affects androgen output, and whether estrogen synthesis (and its inhibition) occurs mainly within the tumour or benign tissue (breast and elsewhere), the effects on intratumour estrogen levels would be similar. However, the antitumour effect of oophorectomy in premenopausal patients indicates circulating E2 of ovarian origin to be of key importance to tumour growth. Regarding postmenopausal women, the fact that plasma E2 levels in the upper compared to lower quartile is associated with an elevated risk for breast cancer of 3–5 fold Citation[23] underlines the clinical importance of total body estrogen synthesis to tumour growth also among postmenopausals. In a recent study, we confirmed high intra-tumour levels of E2 to be limited to ER positive tumours only. In contrast, we found E1 levels to be lower in tumour compared to benign breast tissue in pre- and postmenopausal women, independent of tumour ER status Citation[24]. Thus, alternative explanations, like intra-tumour reduction of E1 into E2, or enhanced receptor ligand binding in ER+ tumours, may explain high levels of E2 in ER+ tumours.
These findings have significant implications not only to our understanding of the endocrine mechanisms regulating tissue hormone levels but may add to our understanding of resistance to endocrine therapy and thus the mechanism of action of additive treatment with estrogens as well. While short term (3–4 months on treatment) studies applying aromatase inhibitors as pre-surgical therapy has evaluated drug effects on intra-tumour estrogen levels Citation[25–27], there is no evidence from these studies suggesting tumours may ”escape” estrogen suppression through local hormone production within that time interval. These observations therefore are consistent with a mechanism of “cellular adaptation” to low estrogen concentrations by breast cancer cells (see later). Further, the antitumour effects of oophorectomy, but also breast cancer risk related to plasma estrogen levels in postmenopausal women outlines a significant role of circulating estrogens to tumour growth.
Endocrine treatment approaches over the years
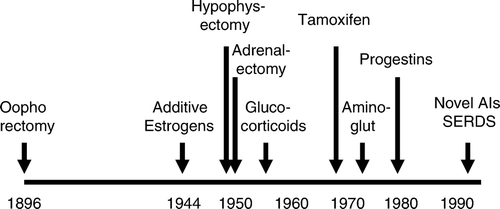
The “time axis” for endocrine therapy development is depicted in . Following the seminal discovery by Beatson of an effect of ovarian ablation in advanced breast cancer Citation[28], this option remained the sole endocrine treatment strategy for half a century. Subsequently, it was followed by estrogens administered in pharmacological doses Citation[29], Citation[30], ablative therapies like hypophysectomy and adrenalectomy Citation[31], Citation[32] and, later, additive therapies including androgens Citation[33], glucocorticoids Citation[34] and progestins at high doses Citation[35]. These options, similar to the first generation aromatase inhibitor aminoglutethimide Citation[36], all revealed anti-tumour effects in advanced disease but at a price of morbidity and toxicity. Thus, they were all successively replaced by contemporary treatment options, including use of SERMS, third-generation aromatase inhibitors, and the SERDS. The effects of the different options regarding cellular hormone disposition and potential mechanisms of action are depicted in .
Figure 2. Potential effects of different endocrine treatment options on hormonal disposition and mechanisms of action in breast cancer cells.
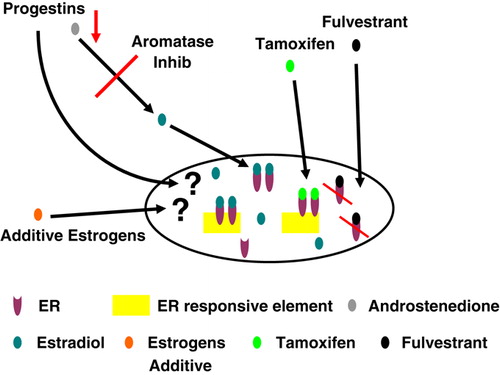
While implementation of progestins at high doses occurred in parallel to the first discoveries of the therapeutic benefits of tamoxifen Citation[37] and the first generation aromatase inhibitor aminoglutethimide Citation[38], Citation[39], the readers are referred to other reviews summarizing early development and clinical use of these compounds Citation[40], Citation[41]; the subject of this review is to explore the potential for a new home for the orphans no longer belonging to the house of contemporary agents. For this purpose, these options will be dealt with under two major headings; ablative, and additive treatment options.
Ablative treatment options
This category includes three options; oophorectomy, adrenalectomy, and hypophysectomy, aiming at eliminating circulating estrogens.
The finding by George Beatson Citation[28] that oophorectomy had dramatic anti-tumour effects in advanced breast cancer introduced a “treatment strategy” in contemporary use more than a century after its implementation. While over the years surgical oophorectomy was replaced by radiological ablation Citation[42] and, more recently, ovarian ablation with use of LH-RH analogues, each strategy implies the same therapeutic principle – removing the main source of E2 in premenopausal women. Yet, there are some minor differences regarding hormonal effects. First, while the estrogen levels drop instantly in response to surgical ablation, for radiological ablation the endocrine effects develop over weeks. Second, LH-RH analogues, in contrast to the previous options, initiate an initial “spike” in plasma FSH and LH levels before generating hormonal suppression Citation[43]. Notably, while LH-RH receptors have been identified in the normal human breast as well as in breast cancer tissue (see references in Citation[44]), their role in tumour growth remains obscure. Thus, in practise, these treatment options are considered similar from a therapeutic point of view, indicating no therapeutic place of surgical oophorectomy in today's armamentarium.
The hypothesis behind surgical adrenalectomy and hypophysectomy was to remove residual estrogen production in postmenopausal women. While subsequent studies identified the adrenal gland as the main source of circulating androgens with estrogen production to take place in peripheral tissue, direct (adrenalectomy) or indirect (hypophysectomy) removal of the androgen source was found to be effective anti-tumour options in postmenopausal women with advanced breast cancer Citation[31], Citation[32], Citation[45], Citation[46].
In theory, there are some differences between the two options, in as much as hypophysectomy, apart from removing ACTH, also eliminates secretion of the gonadotrophins, but in addition prolactin. The potential role of prolactin levels to breast cancer growth remains poorly understood Citation[47]. As for the gonadotrophins, the ovary express a low albeit significant secretion of androgens, revealed by a minor drop in plasma levels of testosterone upon administration of an LH-RH analogue to postmenopausal women Citation[48]. However, as long as no controlled study has evaluated sequential use of the two treatment options, we do not know whether any of these endocrine differences may play a role to their anti-tumour effects.
Direct Citation[46], Citation[49–51] as well as indirect Citation[45] comparison suggest a response rate to adrenalectomy and hypophysectomy resembling what is achieved by contemporary therapies. This finding may not be taken as evidence of a complete cross-resistance in-between these options. Thus, the finding of lack of cross resistance in between non-steroidal versus steroidal aromatase inhibitors Citation[52] indicates mechanisms apart from what we consider the main mechanism of action in the anti-tumour effects of many compounds.
Adrenalectomy and hypophysectomy are both associated with significant treatment morbidity and mortality; thus, these ablative therapies have no place in current breast cancer treatment. From a historical perspective, however, they remain the treatment options providing the empirical basis for estrogen deprivation as a treatment option in postmenopausal breast cancer patients.
Additive treatment options except for estrogen administration
Attempting to achieve a “medical adrenalectomy”, several investigators Citation[34], Citation[53–55] administered glucocorticoids to patients with advanced breast cancer. While anti-tumour effects were observed, a general impression was that the response rates were not as high as observed with the surgical procedures, although we should admit that no randomised trial containing a sufficient number of patients by today's standards were conducted. Other attempts that prove unsuccessful were use of adrenal enzyme inhibitors as ketoconazole and trilostane Citation[56]. Historically, these findings indirectly made a significant contribution to subsequent development of aromatase inhibitors by leading to a search for more potent enzyme inhibitors. Thus, the first generation aromatase inhibitor, aminoglutethimide Citation[57], was initially developed as an unsuccessful antiepileptic that, due to its adrenotoxic effect, was implemented for breast cancer treatment in an attempt to achieve a medical adrenalectomy Citation[38]. Subsequent studies revealed sustained plasma androgen levels despite estrogen suppression Citation[58], leading to identification of its mechanism of action as an aromatase inhibitor Citation[59].
Another form of additive therapy revealing anti-tumour efficacy in advanced breast cancer is use of synthetic progestogens administered at high doses Citation[60], like medroxyprogesterone acetate 1 000 mg daily or megestrol acetate 160 mg daily. Interestingly, short term (1 year) sequential treatment with tamoxifen followed by megestrol acetate was found as efficient as tamoxifen monotherapy for 1–2 years in the adjuvant setting Citation[61]. While several potential mechanisms of anti-tumour action have been proposed, including reduced cellular estrogen uptake and growth factor interactions Citation[35], interestingly, megestrol acetate was found to suppress adrenal androgen secretion and, subsequently, plasma estrogen levels, by about 80% Citation[21].
The potential importance of estrogen suppression to the anti-tumour effects of progestins remains open. While exemestane works in patients becoming resistant to treatment with progestins Citation[62] it also works in patients developing resistance to aminoglutethimide as well as novel third-generation non-steroidal compounds Citation[63]. On the contrary, while there is evidence indicating responses to megestrol acetate subsequent to aminoglutethimide Citation[64], the small number of observations makes this evidence circumstantial. High dose progestins are associated with side effects Citation[65] as weight gain, and some patients acquire a syndrome of disturbing dyspnoea due to interstitial oedema in the lungs. However, for patients becoming resistant to all forms of endocrine treatment options following initial endocrine responsiveness high dose progestins may be a feasible option, although we should be well aware we lack clinical evidence confirming efficacy in this setting.
Most breast cancers harbour the androgen receptor (AR) at a concentration resembling what is observed for the ER. Thus, about 80% of all breast cancers have been reported to express the AR > 10 fmol/mg protein Citation[66], the limit in general used to define breast cancers as positive with respect to the ER. Androgens are shown to inhibit breast cancer cells in vitroCitation[67] and express anti-tumour effects in human breast cancer Citation[33], Citation[68–70], although for one compound, testololactone, subsequent discovery that this compound in addition was a weak aromatase inhibitor Citation[71] makes interpretation of the clinical results for this compound uncertain.
While androgens administered at higher doses cause substantial side effects (hirsuitism), the question whether androgens at low doses may add benefits in concert to other endocrine treatment options remains open. As mentioned above, patients becoming resistant to non-steroidal aromatase inhibitors may benefit from steroidal aromatase inhibitors like exemestane Citation[52]. While the mechanism beyond lack of cross-resistance between these compounds is incompletely understood, notable exemestane, through its major metabolite 17hydroexemestane, express light androgen-agonistic effects Citation[72], and experimental evidence suggests estrogen deprivation may sensitise breast cancer cells to the growth-inhibitory effects of androgens Citation[73], Citation[74].
In conclusion, except for potential treatment with progestins at high doses, none of the additive therapies summarised here are candidates for contemporary sequential endocrine therapy on their own. An unaddressed question remains, however; whether androgens, administered at low doses, may enhance efficacy of treatment with novel potent aromatase inhibitors.
Estrogens as additive treatment in breast cancer
In 1944, two separate groups reported the efficacy of different synthetic estrogens in advanced breast cancer Citation[29], Citation[30]. The scientific rationale was based partly on findings that carcinogenic hydrocarbons under certain circumstances could generate growth arrest Citation[75]. While diethylstilbestrol became the agent most widely used Citation[30], Citation[76–78], noteworthy responses was found not restricted to this single compound, as triphenylchlorethylene, 16-alpha-estradiol diproprionate and ethinylestradiol all were found to be effective Citation[29], Citation[79–81]. Subsequent studies revealed the effect of estrogens administered in pharmacological doses to be effective not only for postmenopausal breast cancer patients but among premenopausal patients as well Citation[78]. Diethylstilbestrol was administered up to massive doses of 1 500 mg daily Citation[77]. Subsequently, with the introduction of tamoxifen, randomised studies revealed similar response rates but less toxicity for tamoxifen as compared to estrogen therapy Citation[81], Citation[82], leaving estrogen additive treatment out of clinical use for more than two decades. Interestingly, a long-term follow-up on one of these studies Citation[83] revealed improved survival outcome for patients treated with diethylstilbestrol as compared to tamoxifen. Notably, estrogen additive therapy has been associated with increased risk of cardiovascular complications among males treated for prostatic carcinoma Citation[84], and the long-term follow-up report by Peethambaram et al. Citation[83] also confirmed a higher incidence of cardiovascular complications among breast cancer patients treated with diethylstilbestrol as compared to tamoxifen.
In vitro, several groups Citation[85–87] have shown MCF-7 cells gradually exposed to E2 at low concentration over months (so-called Long Term Estrogen Deprivation, LTED) to develop a state of “estrogen hypersensitivity”, in as much as these cells get maximum growth stimulation by E2 at a concentration 1/1 000 to 1/10 000 the concentrations required for optimal growth stimulation of the wild-type cells. The growth stimulation curve for MCF-7 cells is “bell-shaped, meaning that E2 at concentrations above what is needed for optimal stimulation actually suppresses cellular growth. During LTED, this “bell-shaped” curve moves to the left; thus, E2 at a concentration stimulating the growth of wt MCF-7 cells now become growth inhibitory (). Actually, estradiol induces apoptosis in cells becoming resistant to estrogen deprivation Citation[88].
Figure 3. Growth-stimulation curves of wild-type MCF-7 cells and long-term estrogen deprived cells in culture. Notably, following long-term estrogen deprivation, these cells may achieve maximal growth-stimulation by a concentration of 1:1 000 to 1:10 000 concentration required for wild-type cells. Notice the “bell-shaped curve” moves to the left, meaning that estrogens at concentrations causing optimal growth-stimulation in wild-type cells now may inhibit growth of cells undergoing long-term estrogen deprivation. Adapted with permission from reference Citation[86].
![Figure 3. Growth-stimulation curves of wild-type MCF-7 cells and long-term estrogen deprived cells in culture. Notably, following long-term estrogen deprivation, these cells may achieve maximal growth-stimulation by a concentration of 1:1 000 to 1:10 000 concentration required for wild-type cells. Notice the “bell-shaped curve” moves to the left, meaning that estrogens at concentrations causing optimal growth-stimulation in wild-type cells now may inhibit growth of cells undergoing long-term estrogen deprivation. Adapted with permission from reference Citation[86].](/cms/asset/903a9a34-fbc3-4e2d-a3ac-abcb5b32fbb4/ionc_a_411954_f0003_b.jpg)
Several explanations to the phenomenon of LTED has been proposed including activation of the ERK MAP kinase and the PI3kinase/mTOR pathways, non-genomic effects of E2, or activation of growth factor receptors or proto-oncogenes as IGF-IR, EGFR or HER-2; the readers are referred to several excellent reviews on this issue Citation[89–91]. Whatever the role of each mechanism, it seems clear that LTED is not due to upregulation of the ER level; rather, it seems to be related to enhancement of co-activators, downstream effector mechanisms or interacting growth stimulatory pathways.
While LTED is an in vitro finding, clinical observations suggest similar mechanisms may cause acquired resistance to estrogen suppression in vivo. Thus, it may explain why postmenopausal patients achieve a response to endocrine manipulation equal to what is observed among premenopausal patients despite differences with respect to plasma and tissue estrogen levels. It may also explain why premenopausal patients developing acquired resistance to ovarian ablation may subsequently respond to further estrogen deprivation with use of aromatase inhibitors, and how postmenopausal patients with very low plasma estrogen levels due to previous adrenal- or hypophysectomy () may subsequently benefit from an aromatase inhibitor Citation[92]. Potentially, it may also explain the anti-tumour effects of estrogens administered at pharmacological doses.
Figure 4. “The estrogen suppression paradox”. This figure illustrates average plasma estradiol levels in premenopausal women and after ovarian ablation (left), normal postmenopausal women and after exposure to aromatase inhibitors (middle), and patients previously having estrogen suppression through adrenalectomy or hypophysectomy Citation[92] exposed to aromatase inhibitors (right).
![Figure 4. “The estrogen suppression paradox”. This figure illustrates average plasma estradiol levels in premenopausal women and after ovarian ablation (left), normal postmenopausal women and after exposure to aromatase inhibitors (middle), and patients previously having estrogen suppression through adrenalectomy or hypophysectomy Citation[92] exposed to aromatase inhibitors (right).](/cms/asset/7475a48b-91c7-4945-b61a-69a194b160d1/ionc_a_411954_f0004_b.jpg)
Notably, in case LTED may develop in vivo in response to aromatase inhibition, this could implicate tumours developing acquired resistance to aromatase inhibition may actually be sensitised to treatment with additive estrogens. Based on this hypothesis, together with Professor Howells group in Manchester, we treated a total of 32 patients with metastatic breast cancer heavily exposed to endocrine treatment with diethylstilboestrol 15 mg daily Citation[3], the dose in general use. Of these patients, ten of 32 obtained an objective response to treatment, with an additional two patients having stable disease > six months. One of the patients (AO) who achieved a complete response of a 16×16 mm cytological confirmed chess wall relapse, received DES treatment for five years, where after she been subject to regular follow-up without active treatment. To this day, she remains disease-free 10 years and six months after commencing DES treatment. In search for alternative endocrine mechanisms explaining this observation, we found DES to decrease plasma androgen and estrogen concentrations but to have no influence on in vivo aromatization Citation[93] probably indicating an effect on adrenal steroid secretion. Yet, it is unlikely these endocrine effects may be of significant importance to the anti-tumour effect observed.
In a second study, Ellis et al. Citation[4] randomised 66 patients with metastatic breast cancer previously exposed to an aromatase inhibitor within < 24 weeks prior to commencing therapy to estradiol 6 mg versus 30 mg daily. They obtained one PR and seven SD among 32 patients in the 30 mg arm with corresponding figures of three PR and seven SD among 34 patients in the 6 mg arm. Thus, “clinical benefit (PR + SD) was 25% versus 29%, respectively. A retrospective analysis from Dr. C. Vogel's team Citation[94] further corroborate the positive results. In addition, there are several ongoing studies expected to report their data in the near future, addressing the issue of efficacy and toxicity in respect to the different dose levels. The finding that patients may respond to a dose of E2 as low as 6 mg daily is of particular interest. Thus, administration of E2 at a dose of 2 mg daily to postmenopausal women Citation[95] may cause plasma levels in the normal premenopausal range (but on average only about 50% of normal levels in smokers), indicating this 6 mg dose may produce plasma levels in the upper physiological range for premenopausal women.
Conclusive remarks
While some uncertainty exists regarding a potential therapeutic role for progestins in pharmacological doses and androgen low doses as supplement to aromatase inhibition, additive treatment, similar to ablative procedures, seems to have a limited place in contemporary endocrine therapy of advanced breast cancer. One exception is administration of estrogens in pharmacological doses. This treatment option now goes through a renaissance, although we should be aware its indication has changed, now becoming a sequential treatment option subsequent to an aromatase inhibitor. Notably, in one study Citation[3] patients had been heavily exposed to multiple endocrine treatments (median of four previous regimens), and all except for four of these patients had received previous treatment with tamoxifen or the SERM droloxifene (8/10 patients with an objective response had previously been treated with a SERM) in addition.
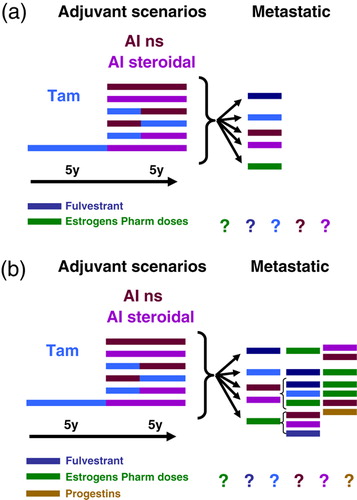
Despite improvement in adjuvant endocrine therapy, many patients harbouring hormone-sensitive tumours will relapse and, thus, be in need of endocrine therapy in advanced disease. While multiple treatment alternatives exist for first-line therapy (a), many of these patients may qualify for subsequent second- or third-line therapy and even endocrine treatment beyond that stage (b). Most likely, we may not see clinical trials outlining an optimal scheme for sequential treatment beyond first-line therapy in the metastatic setting. However, while we do not so far have randomised data confirming superiority for estrogens versus example a SERM among heavily pre-treated patients failing an aromatase inhibitor and more data are warranted, clinical efficacy and toxicity data makes estrogen therapy a reasonable choice in this setting. As for side effects, while estrogen additive therapy may be associated with more side effects as compared to contemporary treatment options, clearly many patients will experience a significantly better quality of life during treatment with such regimens as compared to chemotherapy. With respect to optimal dose scheduling, results from further studies are on their way Citation[89].
Figure 5. a) Clinical scenario for most postmenopausal patients harbouring ER+ tumours relapsing after adjuvant therapy involving aromatase inhibitors. We lack data whether tamoxifen or an aromatase inhibitor should be re-implemented in this setting or the patients alternatively should be exposed to compounds as fulvestrants or estrogen in pharmacological doses. b) The scenario become even more complicated considering second and potential third-line endocrine therapy; in this setting, there is no scientific evidence suggesting the one sequence as compared to the others.
Acknowledgements
Declaration of interest: The author has received speaker's honoraria and participated in Advisory Boards for Pfizer Inc, Astra-Zeneca and Novartis, the main manufacturers of aromatase inhibitors. No conflict of interest with respect to estrogen therapy has been declared.
References
- Iveson TJ, Ahearn J, Smith IE. Response to third-line endocrine treatment for advanced breast cancer. Eur J Cancer 1993; 29A: 572–4
- Ingle JN, Johnson PA, Suman VJ, Gerstner JB, Mailliard JA, Camoriano JK, et al. A randomized phase II trial of two dosage levels of letrozole as third-line hormonal therapy for women with metastatic breast carcinoma. Cancer 1997; 80: 218–24
- Lønning PE, Taylor PD, Anker G, Iddon J, Wie L, Jørgensen LM, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine treatment. Breast Cancer Res Treat 2001; 67: 111–6
- Ellis MJ, Dehdahti F, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Jeffe DB, et al. A randomized phase 2 trial of low dose (6 mg daily) versus high dose (30 mg daily) estradiol for patients with estrogen receptor positive aromatase inhibitor resistant advanced breast cancer. Cancer Res 2009; 69: 67S–68S
- Steinach, E, Kun, H. Transformation of male sex hormones into a substance with the action of a female hormone. Lancet 1937; II:845.
- Jensen, EV, DeSombre, ER, Jungblut, PP. Estrogen receptors in hormone-responsive tissues and tumors. RW Wissler, TL Dao, S Wood, Jr. Endogenous factors influencing host-tumor balance. University of Chicago Press; 1967; 15–30.
- McGuire WL. Steroid receptors in human breast cancer. Cancer Res 1978; 38: 4289–91
- Talman MLM, Rasmussen BB, Andersen J, Christensen IJ. Estrogen receptor analyses in the Danish Breast Cancer Cooperative Group. History, methods, prognosis and clinical implications. Acta Oncol 2008; 47: 789–94
- Rose, C, Thorpe, SM, Andersen, KW, Pedersen, BV, Mouridsen, HT, Blichert-Toft, M, , et al. Beneficial effect of adjuvant tamoxifen therapy in primary breast cancer patients with high oestrogen receptor values. Lancet 1985:16–9.
- Degenshein GA, Bloom N, Tobin E. The value of progesterone receptor assays in the management of advanced breast cancer. Cancer 1980; 46: 2789–93
- McGuire WL, Horwitz KB, Pearson OH, Segaloff A. Current status of estrogen and progesterone receptors in breast cancer. Cancer 1977; 39: 1934–47
- Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Modern Pathol 1998; 11: 155–68
- Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 1999; 17: 1474–81
- Horwitz KB, McGuire WL. Predicting response to endocrine therapy in human breast cancer: A hypothesis. Science 1975; 189: 726–7
- Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 2003; 21: 1973–9
- Lønning PE, Helle SI, Johannessen DC, Ekse D, Adlercreutz H. Influence of plasma estrogen levels on the length of the disease-free interval in postmenopausal women with breast cancer. Breast Cancer Res Treat 1996; 39: 335–41
- Suzuki M, Ishida H, Shiotsu Y, Nakata T, Akinaga S, Takashima S, et al. Expression level of enzymes related to in situ estrogen synthesis and clinicopathological parameters in breast cancer patients. J Ster Biochem Mol Biol 2009; 113: 195–201
- Tseng L, Mazella J, Lee LY, Stone ML. Estrogen sulfatase and estrogen sulfotransferase in human primary mammary carcinoma. J Steroid Biochem 1983; 19: 1413–7
- Lønning PE, Dowsett M, Powles TJ. Postmenopausal estrogen synthesis and metabolism: Alterations caused by aromatase inhibitors used for the treatment of breast cancer. J Steroid Biochem 1990; 35: 355–66
- Couzinet B, Meduri G, Lecce MG, Young J, Brailly S, Loosfelt H, et al. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab 2001; 86: 5060–6
- Lundgren S, Helle SI, Lønning PE. Profound suppression of plasma estrogens by megestrol acetate in postmenopausal breast cancer patients. Clin Cancer Res 1996; 2: 1515–21
- van Landeghem AJJ, Poortman J, Nabuurs M, Thijssen JHH. Endogenous concentration and subcellular distribution of estrogens in normal and malignant breast tissue. Cancer Res 1985; 45: 2900–4
- Dorgan JF, Longcope C, Franz C, Stanczyk FZ, Chang LC, Stephenson HE, et al. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst 2002; 94: 606–16
- Lønning, PE, Helle, H, Duong, NK, Ekse, D, Aas, T, Geisler, J. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J Steroid Biochem Mol Biol 2009;117:31–41.
- Miller WR, Telford J, Love C, Leonard RCF, Hillier S, Gundacker H, et al. Effects of letrozole as primary medical therapy on in situ oestrogen synthesis and endogenous oestrogen levels within the breast. Breast 1998; 7: 273–6
- Geisler J, Detre S, Berntsen H, Ottestad L, Lindtjørn B, Dowsett M, et al. Influence of neoadjuvant anastrozole (Arimidex) on intratumoral estrogen levels and proliferation markers in patients with locally advanced breast cancer. Clin Cancer Res 2001; 7: 1230–6
- Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbo Y, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clinical Cancer Res 2008; 14: 6330–5
- Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma. Suggestions for a new method of treatment with illustrative cases. Lancet 1896; 2: 104–7
- Haddow A, Watkinson JM, Paterson E. Influence of synthetic oestrogens upon advanced malignant disease. Brit Med J 1944; 2: 393–8
- Binnie GG. Regression of tumors following treatment by stilboestrol and x-ray therapy, with notes on case of breast tumour which regressed with stilboestrol alone. Brit J Radiol 1944; 17: 42–5
- Luft R, Olivecrona H, Sjögren B. Hypophysektomy in man. Nord Med 1952; 14: 351–4
- Dao TL, Huggins C. Bilateral adrenalectomy in the treatment of cancer of the breast. Arch Surg 1955; 71: 645–57
- Nevinny HB, Haines CR, Dederick MM, Hall TC. Comparative study of 6-dehydro-17alpha-methyltestosterone + testosterone propionate in human breast cancer. Cancer 1964; 17: 95–9
- Lemon HM. Prednisone therapy of advanced mammary cancer. Cancer 1959; 12: 93–107
- Lundgren S. Progestins in breast cancer treatment. Acta Oncol 1992; 31: 709–22
- Santen RJ, Worgul TJ, Lipton A, Harvey H, Boucher A. Aminoglutethimide as treatment of postmenopausal women with advanced breast carcinoma. Ann Intern Med 1982; 96: 94–101
- Cole MP, Jones CTA, Todd IDH. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Brit J Cancer 1971; 25: 270–5
- Cash R, Brough AJ, Cohen MNP, Satoh PS. Aminoglutethimide (Elipten-Ciba) is an inhibitor of adrenal steroidogenesis: Mechanism of action and therapeutic trial. J Clin Endocrinol Metab 1967; 27: 1239–48
- Santen RJ, Lipton A, Kendall J. Successful medical adrenalectomy with aminoglutethimide. JAMA 1974; 230: 1661–5
- Jordan VC. Biochemical pharmacology of antiestrogen action. Pharmacol Rev 1984; 36: 245–76
- Santen RJ, Manni A, Harvey H, Redmond C. Endocrine treatment of breast cancer in women. Endocrine Rev 1990; 11: 221–65
- Clarke M, Collins R, Davies C, Godwin J, Gray R, Peto R, et al. Ovarian ablation in early breast cancer: Overview of the randomised trials. Lancet 1996; 348(9036)1189–96
- Nillius SJ. The therapeutic uses of gonadotrophin-releasing hormone and its analogues. Clinical Endocrinology I, C Beardwell, GL Robertson. Butterworths, London 1981; 211–37
- Lønning PE, Lien E. Mechanisms of action of endocrine treatment in breast cancer. Crit Rev Oncol/Haematol 1995; 21: 158–93
- Fracchia, AA, Randall, HT, Harrow, JH. The results of adrenalectomy in advanced breast cancer in 500 consecutive patients. Surg Gynecol Obstetr 1967:747–56.
- Fracchia, AA, Farrow, JH, Miller, TR, Tollefsen, RH, Greenberg, EJ, Knapper, WH. Hypophysectomy as compared with adrenalectomy in the treatment of advanced carcinoma of the breast. Surg Gynecol Obst 1971:241–6.
- Bonneterre J, Peyrat J, Demaille A. Is human breast cancer prolactin-dependent?. Rev Endocrine-Related Cancer 1990; 35: 25–30
- Dowsett M, Cantwell B, Lal A, Jeffcoate SL, Harris AL. Suppression of postmenopausal ovarian steroidogenesis with the luteinizing hormone-releasing hormone agonist goserelin. J Clin Endocrinol Metab 1988; 66: 672–7
- Newsome HH, Brown PW, Terz JJ, Lawrence WJ. Medical and surgical adrenalectomy in patients with advanced breast carcinoma. Cancer 1977; 39: 542–6
- Santen RJ, Worgul TJ, Samojlik E, Interrante A, Boucher AE, Lipton A, et al. A randomized trial comparing surgical adrenalectomy with aminoglutethimide plus hydrocortisone in women with advanced breast cancer. N Engl J Med 1981; 305: 545–51
- Wells SAJ, Worgul TJ, Samojlik E, Boucher AE, Lipton A, Harvey H, et al. Comparison of surgical adrenalectomy to medical adrenalectomy in patients with metastatic carcinoma of the breast. Cancer Res 1982; 42(Suppl)3454s–7s
- Lønning PE. Lack of complete cross-resistance between different aromatase inhibitors; a real finding in search for an explanation?. Eur J Cancer 2009; 45: 527–35
- Taylor SG, Ayer JP, Morris RS. Cortical steroids in treatment of cancer. JAMA 1956; 144: 1058–64
- Kofman S, Nagamani D, Buenger RF, Taylor SG. The use of prednisolone in the treatment of disseminated breast carcinoma. Cancer 1958; 11: 226–32
- NissenMeyer R, Vogt JH. Cortisone treatment of metastatic breast cancer. Acta Unio Int Contra Cancrum 1959; 15: 1140–4
- Lønning PE. New endocrine drugs for treatment of advanced breast cancer. Acta Oncol 1990; 29: 379–86
- Lønning PE, Kvinnsland S. Mechanisms of action of aminoglutethimide as endocrine therapy of breast cancer. Drugs 1988; 35: 685–710
- Samojlik E, Veldhuis JD, Wells SA, Santen RJ. Preservation of androgen secretion during estrogen suppression with aminoglutethimide in the treatment of metastatic breast carcinoma. J Clin Invest 1980; 65: 602–12
- Santen RJ, Santner S, Davis B, Veldhuis J, Samojlik E, Ruby E. Aminoglutethimide inhibits extraglandular estrogen production in postmenopausal women with breast carcinoma. J Clin Endocrinol Metab 1978; 47: 1257–65
- Lundgren S, Gundersen S, Klepp R, Lønning PE, Lund E, Kvinnsland S. Megestrol acetate versus aminoglutethimide for metastatic breast cancer. Breast Cancer Res Treat 1989; 14: 201–6
- Andersen J, Kamby C, Ejlertsen B, Cold S, Ewertz M, Jacobsen EH, et al. Tamoxifen for one year versus two years versus 6 months of Tamoxifen and 6 months of megestrol acetate: A randomized comparison in postmenopausal patients with high-risk breast cancer (DBCG 89C). Acta Oncol 2008; 47: 718–24
- Jones S, Vogel C, Arkhipov A, Fehrenbacher L, Eisenberg P, Cooper B, et al. Multicenter, phase II trial of exemestane as third-line hormonal therapy of postmenopausal women with metastatic breast cancer. J Clin Oncol 1999; 17: 3418–25
- Lønning PE, Bajetta E, Murray R, TubianaHulin M, Eisenberg PD, Mickiewicz E, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: A phase II trial. J Clin Oncol 2000; 18: 2234–44
- Brufman G, Isacson R, Haim N, Gez E, Sulkes A. Megestrol-acetate in advanced breast-carcinoma after failure to tamoxifen and/or aminoglutethimide. Oncology 1994; 51: 258–61
- Aisner J, Tchekmedyian NS, Moody M, Tait N. High-dose megestrol acetate for the treatment of advanced breast cancer: Dose and toxicities. Sem Hematol 1987; 24(2,Suppl. 1)48–55
- Lea OA, Kvinnsland S, Thorsen T. Improved measurement of androgen receptors in human breast cancer. Cancer Res 1989; 49: 7162–7
- Ando S, De Amicis F, Rago V, Carpino A, Maggiolini M, Panno ML, et al. Breast cancer: From estrogen to androgen receptor. Mol Cell Endocrinol 2002; 193: 121–8
- Nosaquo ND. Androgens and estrogens in the treatment of disseminated mammary carcinoma. JAMA 1960; 172: 135–47
- Segaloff A, Meyer KK, Cuningha M, Weeth JB. Hormonal therapy in cancer of breast. 23. Effect of 7alpha-methyl-19-nortestosterone acetate + testosterone propionate on clinical course + hormonal excretion. Cancer 1964; 17: 1248
- Volk H, Deupree RH, Goldenberg IS, Wilde RC, Carabasi RA, Escher GC. A dose response evaluation of delta-1-testololactone in advanced breast cancer. Cancer 1974; 33: 9–13
- Barone RM, Shamonki IM, Siiteri PK, Judd HL. Inhibition of peripheral aromatization of androstenedione to estrone in postmenopausal women with breast cancer using Ð1-testoloclactone. J Clin Endocrinol Metab 1979; 49: 672–6
- Johannessen DC, Engan T, Salle Ed, Zurlo MG, Paolini J, Ornati G, et al. Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: A phase I study. Clin Cancer Res 1997; 3: 1101–8
- Macedo LF, Guo ZY, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res 2006; 66: 7775–82
- Suzuki T, Miki Y, Moriya T, Akahira JI, Ishida T, Hirakawa H, et al. 5 alpha-reductase type 1 and aromatase in breast carcinoma as regulators of in situ androgen production. Int J Cancer 2007; 120: 285–91
- Haddow A. Influence of certain polycyclic hydrocarbons on the growth of the jensen rat sarcoma. Nature 1935; 136: 868–9
- Kennedy BJ, Nathanson IT. Effects of intensive sex steroid hormone therapy in advanced breast cancer. JAMA 1953; 152: 1135–41
- Carter AC, Sedransk N, Kelley RM, Ansfield FJ, Ravdin RG, Talley RW, et al. Diethylstilbestrol: Recommended dosages for different categories of breast cancer patients. JAMA 1977; 237: 2079–85
- Kennedy BJ. Massive estrogen administration in premenopausal women with metastatic breast cancer. Cancer 1962; 15: 641–8
- Feldman EB, Silverstein JN, Nayak RV, Carter AC. Antitumor endocrine and metabolic effects of 16alpha-estradiol dispropionate in women with breast cancer. Cancer 1962; 15: 1073–5
- Heuson, JC, Engelsman, E, Blank-van der Wijst, J, Maass, H, Drochmans, A, Michel, J,, et al. Comparative trial of nafoxidine and ethinyloestradiol in advanced breast cancer: An E.O.R.T.C. study. Brit Med J 1975:711–3.
- Matelski H, Huberman M, Zipoli T, Greene R, Lokich J. Randomized trial of estrogen vs. tamoxifen therapy for advanced breast cancer. Am J Clin Oncol 1985; 8: 128–33
- Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, et al. Randomized clinical trial of diethylstilstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med 1981; 304: 16–21
- Peethambaram PP, Ingle JN, Suman VJ, Hartmann LC, Loprinzi CL. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer. An updated analysis. Breast Cancer Res Treat 1999; 54: 117–22
- Mikkola A, Aro J, Rannikko S, Oksanen H, Ruutu M. Cardiovascular complications in patients with advanced prostatic cancer treated by means of orchiectomy or polyestradiol phosphate. Scand J Urol Nephrol 2005; 39: 294–300
- Lippman M, Bolan G, Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res 1976; 36: 4595–601
- Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab 1995; 80: 2918–25
- Dowsett M, Martin LA, Smith I, Johnston S. Mechanisms of resistance to aromatase inhibitors. J Steroid Biochem Mol Biol 2005; 95: 167–72
- Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li TY, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Nat Cancer Inst 2005; 97: 1746–59
- Jordan VC. The 38th David A. Karnofsky lecture: The paradoxical actions of estrogen in breast cancer – survival or death?. J Clin Oncol 2008; 26: 3073–82
- Osborne CK, Schiff R. Aromatase inhibitors: Future directions. J Steroid Biochem Mol Biol 2005; 95: 183–7
- Yue W, Fan P, Wang JP, Li YB, Santen RJ. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol 2007; 106: 102–10
- Samojlik E, Santen RJ, Worgul TJ. Suppression of residual oestrogen production with aminoglutethimide in women following surgical hypophysectomy or adrenalectomy. Clin Endocrinol 1984; 20: 43–51
- Geisler J, Haynes B, Anker G, Helle H, Ekse D, Dowsett M, et al. Treatment with high-dose estrogen (diethylstilbestrol) significantly decreases plasma estrogen and androgen levels but does not influence in vivo aromatization in postmenopausal breast cancer patients. J Steroid Biochem Mol Biol 2005; 96: 415–22
- Mahtani RL, Stein A, Vogel CL. High dose estrogen as a salvage hormonal strategy for highly refractory metastatic breast cancer (MBC): “Back to the future”. Cancer Res 2009; 69: 386S–7S
- Helle SI, Omsjø IH, Hughes SCC, Botta L, Hüls G, Holly JMP, et al. Effects of oral and transdermal oestrogen replacement therapy on plasma levels of insulin-like growth factors and IGF binding proteins 1 and 3: A cross-over study. Clin Endocrinol 1996; 45: 727–32