To the Editor
Medullary thyroid carcinoma (MTC) represents approximately 5% of all thyroid carcinomas [Citation1]. In contrast to papillary and follicular thyroid cancers which arise from thyroid hormone-producing cells, MTC originates from calcitonin-producing parafollicular C cells in the thyroid [Citation2]. Mutations in the RET proto-oncogene are present in up to 95% of the less-common familial MTC and are seen in over 50% of sporadic cases [Citation3,Citation4]. Diagnostic testing for calcitonin or RET mutations is of limited use in the early detection of MTC in patients without a family history of MTC. Only 15–30% of patients with advanced or metastatic MTC exhibit a response to conventional treatment with doxorubicin, either as a single agent or in combination with cisplatin plus 5-fluorouracil [Citation5]. Overall, 10-year survival rates are 70% when MTC has invaded cervical lymph nodes and 20% when the disease has metastasized to distant sites.
Here, we report a rare case of advanced metastatic MTC that responded to sorafenib, a multikinase inhibitor. A 39-year-old African male presented with a diagnosis of poorly differentiated metastatic neuroendocrine carcinoma at the University of Minnesota Masonic Cancer Center in December 2007. His history with MTC most likely originated in 1990 while he resided in Africa. Past medical history included treatment for schistosomiasis and episodes of hematuria. He was found to have cervical lymph node enlargement, but screening for tuberculosis, HIV-1 and 2, and syphilis was negative. In February 2004, while in the United States, he was found to have a large left supraclavicular soft tissue mass. A restaging CT scan showed multiple metastases within the right lung and mediastinum. In March 2004, cervical lymph node dissection was performed, and a diagnosis of poorly differentiated small cell neuroendocrine carcinoma was made. The patient was treated with cisplatin and etoposide plus radiation to the left upper supraclavicular area. Disease control was maintained until he presented with profuse diarrhea and hypercalcitoninemia in 2007, at which point new metastatic lesions were discovered in the liver and bones, as well as disease progression in the lungs. Therapy with temozolo-mide and bevacizumab was initiated, but after several cycles of this regimen disease progressed.
In December 2007, the patient arrived at the University of Minnesota Masonic Cancer Center for treatment. His medication consisted of octreotide and opium for controlling his hypercalcitoninemia/hypocalcaemia and diarrhea. The patient's response to typical therapy for small-cell neuroendocrine carcinoma was not satisfactory. The decision was made to start sunitinib based on the noted response of neuroendocrine tumors to sunitinib in a phase 2 randomized trial [Citation6], as well as our own experience. A CT/PET scan performed on May 22, 2008 showed improvement of metastatic disease in the media stinum and thoracolumbar vertebral column. No significant change was seen in bilateral pulmonary metastases or periaortic lymph nodes. Worsening disease was also noted in the left axilla, liver and sternum.
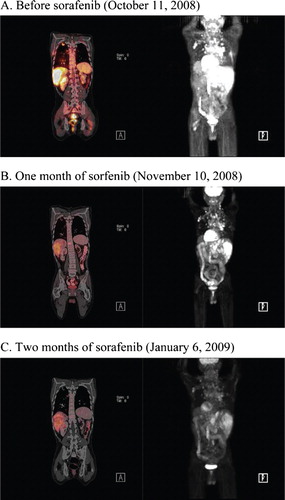
Following the cessation of sunitinib in August 2008, sorafenib was started at 400 mg twice daily in an effort to prevent tumor progression and mitigate sunitinib-related fatigue and mucosal ulcerations. After one month of treatment, sorafenib was discontinued for five weeks because of overwhelming fatigue. However, sorafenib therapy was resumed on October 14, 2008 due to rapidly progressing disease (). Within one month the patient showed an objective response to sorafenib treatment ().
Figure 1. PET scans (left panel) show a pronounced decrease of metabolic activity of metastatic nodules within the patient's bones, lungs and liver. Right panels reveal a dramatic decrease in the size and number of metastatic nodules in the lungs, mediastinum, thoracolumbar vertebral column, periaortic lymph nodes, left axilla, liver and sternum.
In November 2008, the patient's calcium level was low at 4.1 mg/dl, with ionized calcium at 2.8 mg/dl. These results were followed with evaluation of the calcitonin level, which was found to be high at 21 719 pg/ml and two weeks later still higher at 29 682 pg/ml. These tests suggested a diagnosis of medullary thyroid cancer. Because earlier diagnostic tissue from outside the institution was not available for review, an ultrasound-guided fine needle aspiration of the nodule in the right lobe of the thyroid gland was performed and returned a small amount of neoplastic tissue suitable for immunostaining. The tumor showed positive immunoreactivity for calcitonin, chromogranin and synaptophysin, consistent with medullary thyroid carcinoma.
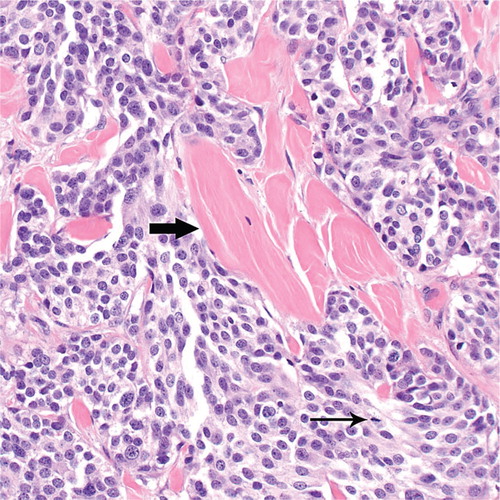
After two months of sorafenib therapy, widespread improvement in metastatic disease was evident on imaging scans (). The calcitonin level had also improved to 23 258 pg/ml. In March 2009, the calcitonin level worsened to 68 969 pg/ml and subcutaneous nodules appeared. Surgical resection of a 1.6 cm tumor metastatic to the skin and subcutaneous soft tissues of the chest was found to be composed of solid nests of medium-sized cells with interspersed bands of acellular eosinophilic material consistent with amyloid (). Tumor cells had moderately pleomorphic nuclei with finely granular (“salt-and-pepper”) chromatin and small nucleoli. These morphologic features are those of medullary thyroid carcinoma. CT and PET scans performed in May 2009 showed disease progression in the cervical, mediastinal, axillary lymph nodes and liver. Sorafenib therapy was stopped after approximately seven months of disease control.
Figure 2. Medullary thyroid carcinoma metastatic to the skin and subcutaneous soft tissues of the chest (March 2009). Notice the bands of amyloid (thick arrow) interspersed between nests of tumor cells. An occasional mitotic figure is seen (thin arrow). Hematoxylin and eosin, original magnification ×400.
Our case shows that sorafenib is potentially promising in MTC. Sorafenib is a small molecule tyrosine kinase inhibitor that effectively targets the angiogenic activity of receptor tyrosine kinases [Citation7,Citation8]. Emerging evidence also suggests that RET onco-genes could be susceptible to targeting via kinase inhibition [Citation9,Citation10]. Sorafenib has already been shown to have efficacy against other neoplastic diseases, and is currently being evaluated in clinical trials for treatment of thyroid carcinomas. Randomized trials for sorafenib are underway that may lead to eventual drug approval for thyroid cancer [Citation11,Citation12]. In our case, calcitonin levels did not correlate with disease response to sorafenib. This observation is consistent with previous reports that this molecular marker does not adequately reflect the dynamics underlying the progression of MTC [Citation13]. Further clinical evaluation is warranted to evaluate the efficacy and mechanism of sorafenib activity in advanced metastatic MTC.
References
- Moley JF. Medullary thyroid cancer. Surg Clin North Am 1995;75:405–20.
- Ball DW. Medullary thyroid carcinoma. Thyroid cancer. A comprehensive guide to clinical management. Wartofsky L. 2000;42:365.
- Hong D, Ye L, Gagel R, Chintala L, El Naggar AK, Wright J, . Medullary thyroid cancer: Targeting the RET kinase pathway with sorafenib/tipifarnib. Mol Cancer Ther 2008;7:1001–6. Epub 2008 Apr 29.
- Mulligan LM, Kwok JBJ, Healey CS, Elsdon MJ, Eng C, Gardner E, . Germline mutation of the ret protoonco-gene in MEN type 2A. Nature 1993;363:458–60.
- Brierley JD, Tsang RW. External radiation therapy of medullary thyroid cancer. Thyroid cancer. A comprehensive guide to clinical management. Wartofsky L, Van Nostrand D. 2006;72:605–7.
- Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, . Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008;26:3403–10.
- Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, . Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 2008;26:4714–9. Epub 2008 Jun 9.
- Lanzi C, Cassinelli G, Nicolini V, Zunino F. Targeting RET for thyroid cancer therapy. Biochem Pharmacol 2009;77:297–309. Epub 2008 Nov 6.
- Plaza-Menacho I, Mologni L, Sala E, Gambacorti-Passerini C, Magee AI, Links TP, . Sorafenib functions to potently suppress RET tyrosine kinase activity by direct enzymatic inhibition and promoting RET lysosomal degradation independent of proteasomal targeting. J Biol Chem 2007;282:29230–40. Epub 2007 Jul 30.
- Henderson YC, Ahn SH, Kang Y, Clayman GL. Sorafenib potently inhibits papillary thyroid carcinomas harboring RET/PTC1 rearrangement. Clin Cancer Res 2008;14:4908–14.
- Sherman SI. Advances in chemotherapy of differentiated epithelial and medullary thyroid cancers. J Clin Endocrinol Metab 2009.
- Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, . Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 2009.
- Ball DW. Medullary thyroid cancer: Therapeutic targets and molecular markers. Curr Opin Oncol 2007;19:18–23.