Abstract
Background. Preoperative radiotherapy reduces recurrence but increases postoperative morbidity. The aim of this study was to explore the effect of radiotherapy in rectal mucosa and rectal tumour extracellular matrix (ECM) by studying enzymes and growth factors involved in ECM remodeling. Materials and methods. Twenty patients with short-term preoperative radiotherapy and 12 control patients without radiotherapy were studied. Biopsies from rectal mucosa and tumour were collected prior to radiotherapy and at surgery. Tissue MMP-1, -2, -9, TIMP-1, uPA, PAI-1, TGF-β1 and calprotectin were determined by ELISA. Biopsies from irradiated and non-irradiated peritoneal areas were also analysed. Results. Radiotherapy increased the tissue levels of MMP-2 and PAI-1 in both the rectal mucosa and tumours while calprotectin and uPA showed an increase only in the mucosa after irradiation. The increase of calprotectin was due to an influx of inflammatory cells as revealed by immunohistochemistry. Prior to irradiation, the tumour tissues had increased levels of MMP-1, -2, -9, total TGF-β1, uPA, PAI-1 and calprotectin compared to mucosa, while TIMP-1 and the active TGF-β1 fraction showed no statistical difference. Conclusions. This study indicates a radiation-induced effect on selected ECM remodeling proteases. This reaction may be responsible for early and late morbidity. Interference of this response might reduce these consequences.
Surgical resection is a prerequisite to cure localized rectal cancer. In addition to surgery, preoperative radiotherapy is often administered to reduce local recurrence and possibly improve survival Citation[1]. Radiotherapy is, however, associated with increased morbidity after surgery Citation[2]. Further knowledge of the cellular and molecular events following irradiation is necessary to allow intervention and thus enable improved treatment.
The extracellular matrix (ECM) consists of collagens, laminins, proteoglycans and fibronectins and is remodeled by among others matrix metalloproteinases (MMPs). MMPs and tissue inhibitor of metalloproteinases-1 (TIMP-1) are a family of zinc-dependent enzymes that are elevated in colorectal cancer tissue and associated with more advanced tumours Citation[3] as well as with reactions in rectal tissue after radiotherapy Citation[4–6]. It has been suggested that MMP-9 levels could be used to predict tumour response to radiotherapy Citation[7].
Transforming growth factor-β1 (TGF-β1) also participates in the remodeling of the ECM, but has many other functions such as suppression of the immune system and regulation of cell growth. The physiological functions of TGF-β1 are carried out after dissociation of the latency-associated peptide to form the active mature TGF-β1. This activation can be elicited by endogenous agents, for example plasmin or MMP-9 but also exogenous factors such as irradiation and radiation-activated TGF-β1 may be involved in the mechanisms behind fibrosis Citation[8]. However, results of clinical studies are conflicting: some studies have found a decrease or no effect on the TGF-β1 levels after radiotherapy Citation[9–11].
The fibrinolytic plasminogen system (PS) is closely linked to TGF-β1 and MMPs through inhibition and activation. The PS consists of two activators; tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) as well as two inhibitors; plasminogen activator inhibitor -1 and -2 (PAI-1 and -2). PAI-1 is pivotal in fibrosis development Citation[12] and the PAI-1 gene is activated by radiotherapy Citation[13]. The plasminogen system is also involved in the response elicited by radiotherapy Citation[14], Citation[15] and is associated with adhesion formation Citation[16], which may result in small bowel obstruction.
The calcium-binding protein calprotectin is present in neutrophils, granulocytes and monocytes. It has been associated with acute and chronic inflammation Citation[17] and is upregulated in cancers indicating a possible role in carcinogenesis Citation[17] as well as postulated to be an inhibitor of MMPs Citation[18]. Faecal calprotectin is elevated in faeces of patients with inflammatory bowel disease and studies of patients undergoing pelvic radiotherapy have evaluated its use as an indicator of radiation-induced proctitis Citation[19], Citation[20]. No studies have examined the levels of calprotectin before and after irradiation in rectal cancer or adjacent mucosa.
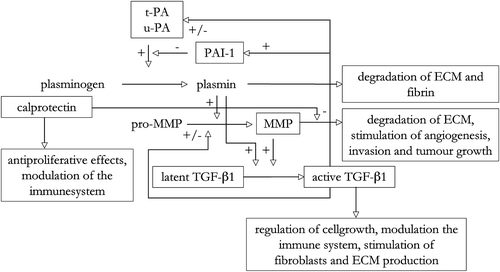
The aim of this study was to prospectively study the effect of radiation therapy on MMP-1, -2 and -9, TIMP-1, TGF-β1, uPA, PAI-1 and calprotectin in rectal mucosa and tumour. In addition the peritoneal response to radiotherapy in regard to MMP-2, -9, TGF-β1, tPA, uPA and PAI-1 was studied. The major interactions of the different proteins described above and analysed in this work are displayed in .
Materials and methods
Study design and patients
This was a prospective study including consecutive patients with localized rectal cancer, scheduled for open surgery, with or without preoperative radiotherapy. Forty-two patients were included, of which 10 patients had to be excluded due to different radiotherapy protocol (two patients), transanal endoscopic surgery (two), neo-adjuvant chemotherapy (two), incomplete biopsies (three) and no surgical treatment (one). Thirty-two patients remained for analysis. Twenty patients received short-term preoperative radiotherapy with a three-field technique, 5x5 gray (Gy). Twelve patients not receiving radiotherapy, but otherwise treated equally served as controls. Inclusion criteria for preoperative radiotherapy were; rectal cancer confirmed by histology, preoperative tumour stage of T3 with possible lymph node involvement, and lowest tumour border less than 10 cm from the anal verge. Preoperative assessment was performed by clinical examination, rigid rectoscopy and, in selected cases, endoscopic ultrasound and/or magnetic resonance imaging (MRI). There was no difference in age (median age 67 (range 36–80) vs. 71 (range 45–88)) or gender (male 65% vs. 58%) between the irradiated and the control group. Surgery using total mesorectal excision (either abdomino-perineal resection or anterior resection) was performed within a median of 3 days (range 3–6) after completion of radiotherapy. No preoperative chemotherapy was given. The patients’ tumours classified according to TNM of the surgical specimens are listed in .
Table I. TNM classification, total number of lymph nodes identified and number of lymph nodes with tumour growth in the intestinal specimens.
Tissue samples
Prior to radiotherapy and surgery baseline biopsies were obtained from both tumour and from adjacent macroscopically tumour-free mucosa using a rigid rectoscope. In the beginning of surgery, peritoneal biopsies from the upper non-irradiated abdomen and from the irradiated pelvic area were collected. Immediately after the rectal specimen was removed, biopsies were collected from the tumour and the rectal mucosa. The mucosal biopsies were obtained within 10 cm from the tumour, within the radiation field Citation[6]. Tissue samples (approximately 20–40 mg wet weight) were immediately snap frozen in liquid nitrogen and stored at −70° until analysis. Biopsies collected from the same areas were immediately fixed in Bouin's solution (Sigma Diagnostic, St Louis, MO, USA) for histology and immunohistochemistry.
Protease and growth factor analysis
Biopsies were mixed in a PBS buffer (phosphate-buffered saline) with NaCl containing 0.01% Triton X-100 to a concentration of 40 mg of tissue/ml of buffer. After homogenization (Ultra Turrax; Janke & Kunkel GmbH, Staufen, Germany) and centrifugation (10 000 g/3 min) the supernatant was stored at −70° C until analysis. MMP-1, -2, -9 and TIMP-1 were analysed using ELISA-kits from Amersham Pharmacia Biotech (Buckinghamshire, UK) Citation[6]. Total, as well as active, TGF-β1, were analysed with ELISA kits from Promega Corporation (Madison WI, USA) Citation[11]. The active fraction of TGF-β1 was assayed directly in the ELISA plate using the kits provided. For measuring the total amount of TGF-β1 additional samples were acidified to pH 3.0 using 1 mol/L HCL, followed by a 15 minute incubation at 22°C, resulting in activation of all TGF-β1. To neutralize samples 1 mol/L NaOH was supplemented before application to the second ELISA plate. The fibrinolytic parameters uPA and PAI-1 were analysed using ELISA-kits from Biopool (Umeå, Sweden) Citation[14]. Calprotectin was analysed using ELISA-kits from Bühlmann Laboratories AG (Schönenbuch, Switzerland). As the technique is not commonly used on homogenized tissue samples, samples with a known high inflammatory response were used as controls and several diluting series were performed.
All results were normalized to total protein content based on the method by Lowry Citation[21], determined by an assay from Bio-RAD (Hercules, CA, USA). There was no difference in total protein content between irradiated and non-irradiated patients. In some patients limited biopsy material restricted the number of analyses. The number of patients included in each analysis is shown in the tables/graphs.
Histological examination and immunohistochemistry
Following fixation, biopsies were washed with PBS, pH 7.4, and dehydrated in increasing ethanol gradients and xylene prior to paraffin embedding. Four to six micrometer sections were deparaffinized and stained with Haematoxylin & Eosin (H&E) for morphologic assessment.
In addition to calprotectin ELISA quantification, the expression of calprotectin in mucosa was evaluated with immunohistochemistry in selected biopsies. Primary mouse anti-human Macrophage L1 protein/Calprotectin antibodies diluted 1:10 (Ab 62227, Lot No: 430744, Abcam, Cambridge, UK) were used together with the DAKO Envision system (DAKO Cytomation, Glostrup, Denmark) and detected with diaminobenzidine. Incubations of tissue sections with mouse IgG directed towards an enzyme neither present nor inducible in mammalian tissue instead of primary antibodies (X-0931, DAKO Cytomation, Glostrup, Denmark) at the same concentration, served as negative controls. Slides were counterstained with Haematoxylin prior to dehydration and mounting with cover slips. Evaluation with distribution and qualitative comparison was performed using Nikon Eclipse 800 microscope together with Nikon Coolpix 995 digital photo equipment (Nikon Instruments Inc, Melville, New York, USA).
Statistical analysis
The Kolgomorov-Smirnov test was used to confirm that most data was not normally distributed. Statistical analysis was performed using Wilcoxon signed rank test, χ2 test, Fishers’ exact test and the Mann-Whitney U analysis. The results are presented as median and interquartile range (IQR) as the distribution was non-parametric. The graphs are presented as box-plots showing the median (horizontal line), IQR (boxes) and 1.5 times IQR (error bars). Statistical analysis was carried out with SPSS 11.0.4 and SPSS 13.0.0 (SPSS Inc., Chicago, Illinois, USA).
Ethical aspects
The University of Gothenburg Ethical committee approved the study.
Results
The expression of ECM remodeling components analysed in irradiated and non-irradiated rectal mucosa, tumour and peritoneal tissues are listed in and . The components analysed showing significant changes after radiotherapy are illustrated in . Altogether 32 patients were studied of which 20 received preoperative radiotherapy and 12 were controls. There were no differences in the analysed components’ levels in the baseline biopsies, prior to therapy, between the irradiated and non-irradiated patients.
Figure 2. Tissue concentrations of MMP-2 (chart A), PAI-1 (B), uPA (C), and calprotectin (D) in irradiated and non-irradiated rectal mucosa and rectal tumours. The number of patients analysed are given below each graph and results are presented as box-plots showing the median (horizontal line), IQR (boxes) 1.5 IQR (error bars).
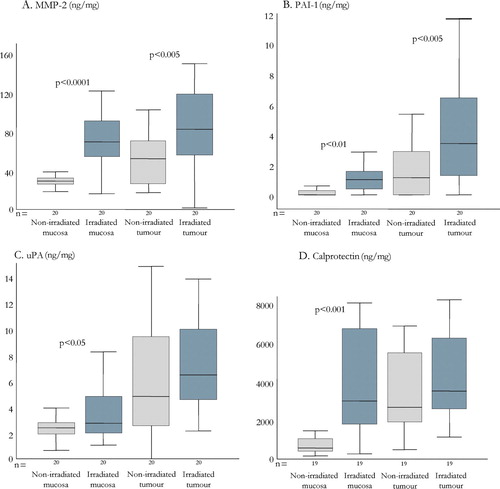
Table II. Proteases and growth factors determined in rectal mucosa and tumour tissue of patients with or without preoperative radiotherapy. Biopsies were collected prior to therapy and at surgery. The results are reported as median with the range in parenthesis. The factors that showed a significant difference are indicated by asterisks (*) and the components showing statistically significant differences are shown as box-plots in Figure II. The reduced sample size in some of the measurements is due to insufficient biopsy material.
Table III. Proteases and growth factors determined in peritoneal tissue biopsies collected at start of surgery. Biopsies were taken from the pelvic area and the upper part of the abdomen outside the radiation treatment field. The results are reported as median and the range (in parenthesis).
Studies of rectal mucosa and tumour
MMPs and TIMP-1
In the baseline biopsies from both groups of patients MMP-1, -2 and -9 were higher in the tumour compared to mucosa, while no differences were seen in TIMP-1 levels (). These findings did not differ between the two groups.
Radiotherapy significantly increased MMP-2 levels in both the rectal mucosa and the tumour tissue (A). No significant changes in the levels of MMP-1, MMP-9 or TIMP-1 upon radiation were found (). In control patients there was a significant reduction in TIMP-1 levels in the biopsies collected during surgery (p < 0.05, n = 12) compared to preoperative biopsies.
Total and active TFG-β1
Total TGF-β1 was higher in tumour compared to mucosa in the baseline biopsies. Neither the total TGF-β1 nor the active form of this cytokine in the mucosa and tumour tissues was affected by irradiation ().
uPA and PAI-1
Both uPA and PAI-1 levels were higher in the baseline tumour tissue compared to the mucosa (). Irradiation resulted in a significant increase of both mucosal and tumour PAI-1 levels (B) while only a slight increase in mucosal uPA and no effect on tumour uPA was seen (C).
Calprotectin
The baseline biopsy levels of calprotectin were significantly higher in the tumour tissues compared to the mucosa (). The levels were significantly increased in the mucosa after radiation treatment while the tumour tissues showed no significant difference (D).
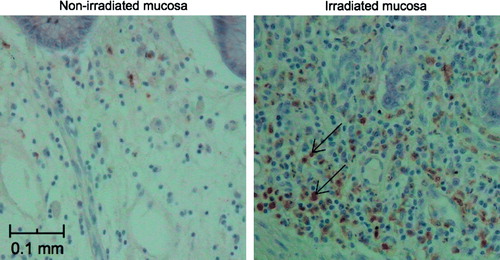
Immunohistochemistry () revealed that the high levels of irradiated mucosal calprotectin are due to an influx of inflammatory white blood cells, such as granulocytes (arrows in ) in lamina propria and submucosa.
Figure 3. Immunohistochemistry of non-irradiated (left chart) and irradiated (right) rectal mucosa Calprotectin is located to inflammatory cells in lamina propria of the irradiated mucosa (arrows). The mucosal epithelial cells and submucosa are negative.
As expected, the irradiated mucosa displayed increased cellularity of the lamina propria with fibroblasts, as well as granulocytes and lymphocytes compared to non-irradiated mucosa. There were also signs of architectural crypt distortion. In the non-irradiated tumour biopsies tumour cells and some inflammatory cells were found. After irradiation, tumour cells were swollen and there were apparent changes in the stroma with an abundance of fibroblasts, granulocytes and lymphocytes.
Expression of ECM remodeling factors in irradiated and non-irradiated peritoneum
Peritoneal biopsies were obtained during surgery from the pelvic region and upper abdominal region outside the radiation field. The results of analysed components are listed in . No differences could be detected between the pelvic and upper abdominal biopsies in neither the non-irradiated control patients nor the irradiated patients. tPA was also analysed in the peritoneal biopsies since this factor has been shown to be of importance for adhesion formation Citation[16]. The levels for non-irradiated pelvic peritoneum were 17.7 ng/mg protein (12.1–22.2) and upper abdomen 14.2 (6.6–20.5). In the upper abdomen peritoneum in irradiated patients the level was 11.4 (6.1–14.2). These figures are not significantly different. However, in irradiated patients, the tPA levels in the pelvic peritoneal biopsies was significantly lower; 7.8 (4.0–16.1; p < 0.05) compared to non-irradiated patients.
Discussion
This study shows that pre-operative radiotherapy for rectal cancer induces changes in tissue levels of several proteases involved in ECM remodeling. Of the MMPs, MMP-2 was increased in irradiated mucosa and tumour tissue while MMP-1 and MMP-9 were not affected. It has been suggested that higher levels of MMP-2 after radiotherapy would facilitate tumour cell dissemination Citation[5]. This is supported by in vitro studies showing an increased invasive potential in tumour cell lines after radiotherapy Citation[22]. Although radiotherapy reduces local recurrence there is a possibility that MMP-2 inhibitors could further improve these results especially in cases where the surgical margin is small. Higher levels of MMP-2 have also been associated with anastomotic dehiscence Citation[23], wound-infections, fistula formation Citation[6] and radiation-induced diarrhoea Citation[4].
Preoperative radiotherapy has been indicated as risk factor for anastomotic leakage Citation[24] although this has not been confirmed by large randomized trials. A limitation with this human study is that biopsies were taken from the removed specimen. Although it is probable that the levels of factors analyzed in the biopsies reflect anastomotic area levels, this cannot be certain. There are indications that timing of surgery after radiotheraphy is important to reduce complication rate Citation[25]. A sub group analysis, showed lower levels of MMP-2 in rectal mucosa in irradiated patients operated three days after radiotherapy compared with patients operated four days after treatment. It is possible that these biopsies reflect the increased remodeling taking place and may indicate the importance of reduced surgical delay after radiotherapy. We found no effect of radiation on MMP-1 or MMP-9 in this study, which is somewhat contradictory to previous findings Citation[4–6]. MMP-9 mRNA seems to increase after a few days after radiotherapy, which could indicate that the reactions of MMP-9 due to irradiation are not fully present at the time of our biopsies Citation[15].
Our study is in accordance with an earlier report that radiotherapy does not affect TIMP-1 in rectal tumours Citation[7]. TIMP-1 has been found to increase shortly after short-term radiotherapy in mucosa in animal studies Citation[15]. The timing of biopsies is important when interpreting TIMP-1 results but we hypothesize that the control group TIMP-1 decrease is attributed to the surgical trauma and that radiotherapy may alter this response. It has been shown that tissue TIMP-1 initially decreases after arterial injury Citation[26], but later the TIMP-1 levels increased.
Faecal calprotectin levels have been found to be higher after radiotherapy in humans although studies are not conclusive Citation[19], Citation[20]. We found increased levels of calprotectin in the irradiated tissue due to an influx of inflammatory cells. Further studies are required to see if mucosal biopsies analysed for calprotectin can be used as an indicator of the extent of radiation reaction and predict subsequent response. Such studies could be performed on archived specimens from randomized trials with patients randomized to surgery alone or addition of preoperative radiotherapy.
Evidence points toward a radiation induced activation of latent TGF-β1 Citation[8] but this activation may only be seen a limited time, which may explain why it was not seen in this study, as patients were operated up to six days after termination of radiotherapy.
The PAI-1 gene is activated by radiotherapy Citation[13] and PAI-1 protein levels are increased in both rectal mucosa and tumour after radiotherapy Citation[14], Citation[15], which is confirmed in this study. PAI-1 participates in the radiotherapy associated fibrosis development Citation[12], and higher levels of uPA and PAI-1 are found in tumour tissue indicating their role in tumour development Citation[14].
We hypothesized that the increased risk of bowel obstruction after surgery combined with radiotherapy Citation[2] could be related to an unfavourable reaction of the peritoneum. We found lower levels of tPA in irradiated peritoneum compared to controls, which could indicate a reduced fibrinolytic activity signifying an increased risk for adhesions Citation[16].
This is one of few human studies on this topic. A difference in TNM-classification between irradiated and non-irradiated patients could be expected due to radiotherapy treatment selection, but our material does not differ significantly in this regard. The use of MRI in the pre-operative setting has improved estimation of tumour stage and selection of patients to radiotherapy, but during the time of this study MRI was not available for all patients, and perhaps this can in part explain this lack of difference. One interesting finding was that the baseline biopsies displayed the same differences between mucosa and tumour as previously shown in operative biopsies except for active TGF-β1 Citation[6], Citation[11], Citation[14], indicating that operative biopsies in many cases can be used reflecting the preoperative setting.
Values are normalized to the total protein content but the biopsies are heterogeneous. However, each of the irradiated patients constitutes its own control, which is the strength of this study. The data from the non-irradiated patients make a reference for the possibility of systematic differences due to anaesthesia, surgery procedures and possibly differing biopsy procedures at diagnoses and at surgery. Since the expression of the various markers change over time post irradiation, the results of this study may over-look early and late changes, which could be studied in patients having other radiotherapy schedules.
In conclusion, although radiotherapy reduces recurrence rates in rectal cancer treatment, it contributes to the increased morbidity following surgery. This study supports our previous data pointing to effects on the levels of MMP-2, PAI-1, uPA by radiotherapy in rectal mucosa and tumour. It is clear that radiotherapy affects remodeling of extracellular matrix and that it also has an effect on the rectal mucosal levels of calprotectin, which hasn't previously been demonstrated in radiotherapy of rectal cancer.
Acknowledgements
The authors are indebted to Dr P. Saksena for the evaluation of histology and immunohistochemistry.
This work was supported by the Swedish Research council/Medicin (no 11612), Gothenburg Medical Society, King Gustav V Jubilée Clinic Cancer Research Foundation, Assar Gabrielsson Research Foundation. None of the authors have any conflicts of interest.
References
- Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005; 23: 5644–50
- Birgisson H, Pahlman L, Gunnarsson U, Glimelius B. Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol 2007; 46: 504–16
- Baker EA, Bergin FG, Leaper DJ. Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging. Br J Surg 2000; 87: 1215–21
- Hovdenak N, Wang J, Sung CC, Kelly T, Fajardo LF, Hauer-Jensen M. Clinical significance of increased gelatinolytic activity in the rectal mucosa during external beam radiation therapy of prostate cancer. Int J Radiat Oncol Biol Phys 2002; 53: 919–27
- Kumar A, Collins HM, Scholefield JH, Watson SA. Increased type-IV collagenase (MMP-2 and MMP-9) activity following preoperative radiotherapy in rectal cancer. Br J Cancer 2000; 82: 960–5
- Angenete E, Langenskiold M, Falk P, Ivarsson ML. Matrix metalloproteinases in rectal mucosa, tumour and plasma: Response after preoperative irradiation. Int J Colorectal Dis 2007; 22: 667–74
- Unsal Kilic D, Uner A, Akyurek N, Erpolat P, Dursun A, Pak Y. Matrix metalloproteinase-9 expression correlated with tumor response in patients with locally advanced rectal cancer undergoing preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007; 67: 196–203
- Bentzen SM. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat Rev Cancer 2006; 6: 702–13
- Johnson LB, Adawi D, Agren MS, Jorgensen LN, Wittgren L, Mattsson S, et al. Combination of pre-operative radiotherapy and surgery suppresses local accumulation of collagen and TGF-beta1 in rats. J Surg Res 2006; 133: 136–42
- Richter KK, Fink LM, Hughes BM, Shmaysani HM, Sung CC, Hauer-Jensen M. Differential effect of radiation on endothelial cell function in rectal cancer and normal rectum. Am J Surg 1998; 176: 642–7
- Angenete E, Langenskiold M, Palmgren I, Falk P, Oresland T, Ivarsson ML. Transforming growth factor beta-1 in rectal tumour, mucosa and plasma in relation to radiotherapy and clinical outcome in rectal cancer patients. Int J Colorectal Dis 2007; 22: 1331–8
- Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest 2000; 106: 1441–3
- Zhao W, Spitz DR, Oberley LW, Robbins ME. Redox modulation of the pro-fibrogenic mediator plasminogen activator inhibitor-1 following ionizing radiation. Cancer Res 2001; 61: 5537–43
- Angenete, E, Langenskiold, M, Palmgren, I, Falk, P, Oresland, T, Ivarsson, ML. uPA and PAI-1 in rectal cancer-relationship to radiotherapy and clinical outcome. J Surg Res 2008.
- Strup-Perrot C, Vozenin-Brotons MC, Vandamme M, Benderitter M, Mathe D. Expression and activation of MMP-2, -3, -9, -14 are induced in rat colon after abdominal X-irradiation. Scand J Gastroenterol 2006; 41: 60–70
- Ivarsson ML, Bergstrom M, Eriksson E, Risberg B, Holmdahl L. Tissue markers as predictors of postoperative adhesions. Br J Surg 1998; 85: 1549–54
- Gebhardt C, Nemeth J, Angel P, Hess J. S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol 2006; 72: 1622–31
- Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol 2001; 54: 289–92
- Larsen A, Bjorge B, Klementsen B, Helgeland L, Wentzel-Larsen T, Fagerhol MK, et al. Time patterns of changes in biomarkers, symptoms and histopathology during pelvic radiotherapy. Acta Oncol 2007; 46: 639–50
- Hille, A, Schmidt-Giese, E, Hermann, RM, Herrmann, MK, Rave-Frank, M, Schirmer, M, , et al. A prospective study of faecal calprotectin and lactoferrin in the monitoring of acute radiation proctitis in prostate cancer treatment. Scand J Gastroenterol 2007:1–7.
- Lowry O, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951; 193: 265–75
- Speake WJ, Dean RA, Kumar A, Morris TM, Scholefield JH, Watson SA. Radiation induced MMP expression from rectal cancer is short lived but contributes to in vitro invasion. Eur J Surg Oncol 2005; 31: 869–74
- Stumpf M, Klinge U, Wilms A, Zabrocki R, Rosch R, Junge K, et al. Changes of the extracellular matrix as a risk factor for anastomotic leakage after large bowel surgery. Surgery 2005; 137: 229–34
- Matthiessen P, Hallbook O, Andersson M, Rutegard J, Sjodahl R. Risk factors for anastomotic leakage after anterior resection of the rectum. Colorectal Dis 2004; 6: 462–9
- Hartley A, Giridharan S, Gray L, Billingham L, Ismail T, Geh JI. Retrospective study of acute toxicity following short-course preoperative radiotherapy. Br J Surg 2002; 89: 889–95
- Zou, Y, Qi, Y, Roztocil, E, Davies, MG. Patterns of gelatinase activation induced by injury in the murine femoral artery. J Surg Res 2008.