Abstract
Aim. The tumour differentiation grade has been shown by numerous multivariate analyses to be a stage-independent prognostic factor in colorectal cancer. The aim of this study was to explore the importance of differentiation grading for the staging of colorectal cancer and how it relates to the components of the TNM system. Material and methods. The study was a retrospective single-centre analysis of all patients undergoing surgical resection for colorectal cancer during the period 2002–2007 (n = 1239). The clinical parameters and pathology data of overall stage, differentiation grade, local tumour (T)-stage and metastasis status (M-stage) were included as well as the lymph node count of both assessed and metastatic nodes. The differentiation grade was correlated with demography, overall stage and each component of the TNM staging system. The correlation between differentiation grade and N-stage was also explored for the separate T-stages. Results. The tumour differentiation grade correlated significantly with the overall TNM stage (p < 0.0001). The grade significantly correlated with the T-stage and the risk of having lymph node metastasis (p < 0.0001). A high grade was associated with a higher positive lymph node count in stage III disease (p < 0.0002). For the T-stages, the risk of node metastasis was significantly linked to the tumour grade. A low grade (G1) T2 had a 17% risk of lymph node metastasis compared to a 44% risk for a high grade (G4) T2. Conclusion. Tumour differentiation is an important prognostic factor. It correlates significantly with the overall stage of the TNM system and also to each of its components. The risk of having lymph node metastasis for each T-stage also correlates with the tumour grade. The findings can be of importance in postoperative risk assessment or when considering local resection procedures like TEM.
Cancer is a heterogeneous genetic and biological disorder [Citation1]. Still, the classification is most commonly based on tumour anatomy. The most widely used system for cancer staging is the TNM tumour classification system [Citation2,Citation3]. It takes into consideration the main parameters of local growth, regional lymph node involvement and presence of distant spread. It is systematic and well established. It is also constantly being refined and is currently in its’ sixth edition. With the increasing understanding of cancer pathology, additions have been suggested to the original three components. Such features, like lymphatic or vascular invasion and tumour budding, can at times be seen in pathology reports and are suggested to have prognostic value even if their true role is yet contested [Citation4,Citation5].
Another such factor is the tumour differentiation grade or the synonymously used histological grade. The grade is a factor that at least in part accounts for the tumour biology and thus the characteristics of the cancer. An often criticised problem of the histology grading is the possible subjectivity and inter-observer variability. Efforts have been made towards a standardised definition and there is a WHO standard. This standard has been adopted by the Swedish Association of Pathology and is incorporated into their quality documents where the grades also are defined (KVAST) [Citation6]. The grading is based on the degree of gland formation, and thus the resemblance to the original tissue, and ranges from well (G1) to poor (G3) or even undifferentiated (G4) as described in .
Table I. Differentiation grade definitions, distribution and demography.
The tumour differentiation grade is, according to several multivariate analyses, a stage-independent prognostic factor in colorectal cancer where a high tumor grade is an adverse prognostic factor [Citation7–9]. The grade also correlates to the frequency of lymph node metastasis in stage III colon cancer [Citation10]. The hypothesis was that the differentiation grade can affect both the overall TNM stage and each of its specific components. The aim of this study was to explore the importance of the differentiation grade for the staging in colorectal cancer and how it relates to the factors of the TNM system. A secondary aim was to study each T-stage and the risk of having lymph node metastasis in relation to the tumour grade.
Material and methods
The study was carried out as a retrospective analysis at a high volume university hospital. All patients treated with full surgical resections for colorectal cancer during the period 2002–2007 were included into the study (n = 1 239). Local resections and polyp cancer-cases were not included. Clinical parameters such as gender, age, diagnosis and tumour location were retrieved along with data on types of treatment including possible emergency procedures. The pathology data of overall stage, differentiation grade, local tumour (T)-stage and metastasis status (M-stage) were also included as well as the lymph node status of both assessed and metastatic nodes (N-stage). The differentiation grade was first correlated to diagnosis, treatment and general demographic factors. Subsequently, it was correlated to overall stage and to each component of the TNM staging system. Lastly, the correlation between the T-stage and the N-stage was related to the differentiation grade.
Statistical analysis
SPSS 13.0 software was used for the statistical analysis and the graphs are from the JMP 4.0/SAS (SAS institute) software. The data was analyzed by distribution statistics and ANOVA for parametric data. For the non-parametric data contingency tables with the χ2 (Pearson or Linear association) test were used. The confidence level was set at 95%.
Results
Patients and treatment
The median age of the patients in the study was 71 years. There was an even gender distribution. Eight hundred and eight patients (65.2%) were treated for colon cancer, with a right hemicolectomy being the most common procedure. Four hundred and thirty one patients (34.8%) were treated for rectal cancer with TME (total mesorectal excison) as the predominant procedure. Two hundred and twenty three of rectal cancer patients were treated with short-term 5 × 5 Gy preoperative radiotherapy. A total of 154 patients (12.4%) were subject to emergency surgery. The overall stage distribution and the demography are presented in .
Table II. Patient demography.
Stage and demography
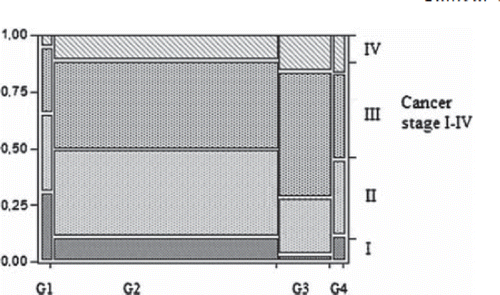
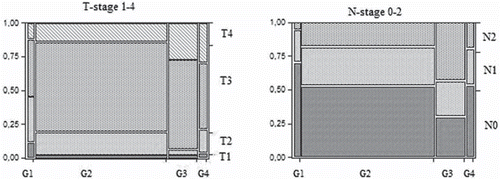
The grade prevalence is detailed in with G2 being most common. There was no correlation between the differentiation grade and the tumour location, diagnosis, age or gender. The median number of assessed lymph nodes overall was 16 (4–101). A lower node assessment rate was associated with preoperative radiotherapy (p < 0.01) and disseminated (stage IV) disease at time of surgery (p < 0.01). There was a significantly higher risk associated with emergency surgery of having higher grade tumours (p < 0.002). The tumour differentiation grade correlated significantly with the overall TNM stage (p < 0.0001). A higher grade was more likely to correspond with a worse overall stage as shown in . The grade also significantly correlated with the T-stage: a high grade being more likely to have a high T-stage (p < 0.0001) (). As indicated by the overall stage, there was a significant correlation between the grade and the risk of having distant metastasis and thus the M-stage ().
Grade and risk of node metastasis
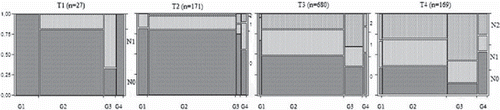
A higher tumour grade correlated with a higher risk of having lymph node metastasis (p < 0.0001) (). In stage III disease, a higher tumour grade was significantly associated with a higher count of tumour involved lymph nodes (p < 0.0002) and thus also of having a N2 node status (). The risk of node positivity was also dependent upon the local T-stage. For each T-stage, the risk of node metastasis was significantly correlated to the tumour grade (). The T1 cancers were rare in our material and few had node metastasis. In T2 the overall risk of node metastasis was 21% (36/171). The low grade (G1) T2 had a 17% risk compared to a 44% risk with high grade (G4, p<0.04). The overall G1 prevalence was 4.4% () whilst in T2 9.9% (n = 17). The high grade tumours (G3/4) had a markedly increased risk of having more than three positive nodes and thus being N2 status. Also in T3/4 tumours the risk of node metastasis was associated with the tumour grade (p < 0.001). In 192 patients, the T-stage was described but not stated. They were treated as a separate unit to avoid misinterpretation bias and had a significant correlation between grade and N-status (p < 0.01).
Discussion
The anatomical extent of the disease is the foundation for the TNM classification and staging system and thus related to the survival prognosis [Citation11]. The differentiation grade is a biologically oriented compliment to the system. In our material, the grade was significantly associated with both the overall stage and with each of the main three TNM-components. This finding could support the hypothesis of a connection between the more biologically oriented grade and the tumour anatomy. The risk of lymph node metastasis has previously been described by several authors. With focus on T1 and T2 tumours the risk has been suggested to be 10–14% and 17–18% respectively [Citation12,Citation13]. Another step for further risk analysis has been the division of the T1 into the submucosal thirds, i.e. the sm1–3 grading, which can then correlate with the risk of node metastasis [Citation14–16]. Choi et al. also reported the cell differentiation as being associated with the risk for node metastasis [Citation14]. Our finding of the close correlation between node status and both T-stage and differentiation concurs with the previous findings. Also, the findings could aid in clarifying the interaction and interdependency between the different factors.
The risk of node metastasis is also of concern in relation to the reliability of the overall staging and the distinction between stages II and III. The staging affects the use of adjuvant chemotherapy which in Sweden is employed in stage III whilst debated for high risk patients of stage II [Citation17]. In identifying the high risk patients there are factors to be considered of which the lymph node assessment itself is one [Citation18]. Even when meeting the assessment standard of 12 lymph nodes there is a risk of a missed positive node, thus an understaging and failure to administer beneficial adjuvant chemotherapy. The data in this material, where high grade tumours of T-stage 3 or 4 had a risk of node metastasis as high as 60–85% () could give some support to consider the grade as a possible risk factor in this context.
The information could also be of importance when considering the use of neoadjuvant chemotherapy. The preoperative work-up can in many instances provide important data. The degree of bowel wall penetration (T-stage) can be assessed with CT/MRI or endorectal ultrasound techniques. A tumour biopsy sample can not only confirm the diagnosis, but also give a hint of the grade. Combined, the data could hypothetically help identify patients at high risk for node metastases that could benefit from this mode of treatment. Yet another setting for preoperative decisions is the question of performing local resections for rectal cancer. With the transanal endoscopic microsurgery (TEM) procedure there is a possibility of full excisions in the rectum [Citation19]. Whilst the advantage is a faster recovery the drawback is risk of recurrence and the lack of information on the lymph node status [Citation20]. However, for selected patients, local resections can be an option if the alternative procedure to TME surgery would carry too high a perioperative risk [Citation21,Citation22]. Our data could support the practice of local procedures for low grade T1 or even T2 cancers, in selected patients, as the risk of node involvement is very low. It should also be clearly noted that the high grade tumours do carry greater risk for node metastasis even for a T2 tumour and thus are not suitable for local procedures.
A problem with the histological grading of colorectal tumours is that it carries some measure of subjectivity and thus is susceptible to interobserver variability [Citation7]. The subjectivity and imprecision in grading may also be related in some degree to tumour heterogeneity. There are structural variations within a tumour. The highest grade found should determine the overall grading, but as tumours can contain heterogenous tissue, sampled areas may not represent the highest grade present. However, the same criticisms could apply to practically all components of the TNM system. The T-stage can differ by a single cell layer and the N-stage is challenged by variables such as the adherence to quality standards and node identification as well as the current discussion on micrometastasis [Citation23]. Therefore, in our opinion, the characteristics and grading classification should not be dismissed on those grounds, but rather be further refined by progressive cooperation with pathologists. A simpler dichotomous system (i.e., low grade and high grade) has been suggested by a multidisciplinary colorectal working group of a consensus conference sponsored by the College of American Pathologists [Citation24]. However, we believe that some important distinctions could be lost in the process. An example would be the low risks associated with low grade tumours and the possibility of identifying patients suitable for local resections. A recent study has also been published on the undifferentiated tumours and their prognosis [Citation25]. Although they carry a worse prognosis, the data in our material does not fully support integration with the poor differentiation of G3. As shown in , the undifferentiated G4 might be more similar, in relation to the TNM-staging, to the medium differentiated G2 than the G3. This could challenge the use of the grade as an ordinal data parameter with the G4 hypothetically being a separate entity.
We believe that this material shows that the tumour differentiation grade is an important prognostic component, which correlates strongly with the three main variables of the TNM tumour classification system. An important finding is that the risk of having lymph node metastasis is correlated not only with the T-stage, but also with the differentiation grade. In the study we chose not to analyze for survival since it can depend on several other factors, such as performance status and treatment eligibility. The factors of the TNM system could in this case be seen as surrogate prognostic markers. As shown the grade correlates with both overall stage and degree of node metastasis and will thus be linked to the survival overall (foremost in stage III/IV). Among the weaknesses of the study are that it is retrospective and the relatively low number of assessed patients. The low prevalence of low grade tumours in the material weakens the results for T2 cancers. Yet, we believe that the findings are of importance and it would be interesting to see if the data can be strengthened by other centres and studies. In our opinion, the drawbacks are balanced by the fact that the patient material is a consecutive unselected series. As it is a single centre material, all patients have been treated along the same guidelines and with a consistent and thorough follow-up. This also concerns the pathology service, where the staff are trained and assessed along the same guidelines. As nodes were a studied factor the node assessment was important and with a median of 16 assessed nodes international standards were well met.
Conclusion
The tumour differentiation is an important prognostic factor. It correlates significantly with the overall stage of the TNM system and also to each of its components. The risk of having lymph node metastasis for each T-stage also correlated significantly with the tumour grade. The findings can be of importance in the postoperative risk assessment for adjuvant chemotherapy. Another possible implication is in preoperative investigation and when considering TEM procedures for rectal cancers.
Acknowledgement
The work was supported by grants from the Swedish Cancer Society, Gothenburg University and the Anna-Lisa and Bror Björnsson Foundation. We would also like to express our gratitude to the staff of our oncology laboratory unit and to Dr Cormac Owens for linguistic support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007;50:113–30.
- UICC/AJCC. TNM classification of malignant tumors. 5th ed. Wiley-Liss, New York 1997.
- Kehoe J, Khatri VP. Staging and prognosis of colon cancer. Surg Oncol Clin N Am 2006;15:129–46.
- Zlobec I, Baker K, Minoo P, Jass JR, Terracciano L, Lugli A. Node-negative colorectal cancer at high risk of distant metastasis identified by combined analysis of lymph node status, vascular invasion, and Raf-1 kinase inhibitor protein expression. Clin Cancer Res 2008;14:143–8.
- Wang LM, Kevans D, Mulcahy H, O'Sullivan J, Fennelly D, Hyland J, . Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 2008.
- SAP, Swedish Association of Pathology: KVAST. National quality guideline documents. Swedish.
- Compton CC. Colorectal carcinoma: Diagnostic, prognostic, and molecular features. Mod Pathol 2003;16:376–88.
- Compton CC. Optimal pathologic staging: Defining stage II disease. Clin Cancer Res 2007;13(22 Pt 2):6862s–70s.
- Mori T, Hirota T, Ohashi Y, Kodaira S. Significance of histologic type of primary lesion and metastatic lymph nodes as a prognostic factor in stage III colon cancer. Dis Colon Rectum 2006;49:982–92.
- Derwinger K, Carlsson G, Gustavsson B. A study of lymph node ratio as a prognostic marker in colon cancer. Eur J Surg Oncol 2008;34:771–5.
- O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420–5.
- Blumberg D, Paty PB, Guillem JG, Picon AI, Minsky BD, Wong WD, . All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum 1999;42:881–5.
- Fang WL, Chang SC, Lin JK, Wang HS, Yang SH, Jiang JK, . Metastatic potential in T1 and T2 colorectal cancer. Hepatogastroenterology 2005;52:1688–91.
- Choi PW, Yu CS, Jang SJ, Jung SH, Kim HC, Kim JC. Risk factors for lymph node metastasis in submucosal invasive colorectal cancer. World J Surg 2008;32:2089–94.
- Nascimbeni R, Burgart LJ Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200–6.
- Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, , The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology 2004;51:998–1000.
- Glimelius B, Dahl O, Cedermark B, Jakobsen A, Bentzen SM, Starkhammar H, . Adjuvant chemotherapy in colorectal cancer: A joint analysis of randomised trials by the Nordic Gastrointestinal Tumour Adjuvant Therapy Group. Acta Oncol 2005;44:904–12.
- Derwinger K, Carlsson G, Gustavsson B. Stage migration in colorectal cancer related to improved lymph node assessment. Eur J Surg Oncol 2007;33:849–53.
- Turner J, Saclarides TJ. Transanal endoscopic microsurgery. Minerva Chir 2008;63:401–12.
- Nascimbeni R, Nivatvongs S, Larson DR, Burgart LJ. Long-term survival after local excision for T1 carcinoma of the rectum. Dis Colon Rectum 2004;47:1773–9.
- Doornebosch PG, Tollenaar RA, De Graaf EJ. Is the increasing role of Transanal Endoscopic Microsurgery in curation for T1 rectal cancer justified? A systematic review. Acta Oncol 2009;48:343–53.
- Baatrup G, Endreseth BH, Isaksen V, Kjellmo A, Tveit KM, Nesbakken A. Preoperative staging and treatment options in T1 rectal adenocarcinoma. Acta Oncol 2009;48:328–42.
- Compton CC. Surgical pathology for the oncology patient in the age of standardization: Of margins, micrometastasis, and molecular markers. Semin Radiat Oncol 2003;13:382–8.
- Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, . Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979–94.
- Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161–8.