Abstract
Background. The use of Intensity-modulated radiotherapy (IMRT) and Helical tomotherapy (HT) is increasing in gynecological cancer patients. No published studies have performed a dosimetric evaluation of whole pelvic radiotherapy (WPRT) using HT for postoperative endometrial cancer. The purpose of this study was to perform a direct dosimetric comparison of three-dimensional conformal radiotherapy (3D-CRT), IMRT and HT plans for WPRT in postoperative endometrial cancer patients, and to evaluate the integral dose to organs at risk (OARs) and normal tissue. Material and methods. We selected ten patients with endometrial cancer undergoing postoperative WPRT. Plans for 3D-CRT, IMRT and HT were developed for each patient. All plans were normalized to deliver 50 Gy to 95% of the PTV. The dosimetry and integral dose to OARs and normal tissue were compared. The significance of differences was tested using a paired two-tailed Student t-test. Results. IMRT were superior to 3D-CRT in dose conformity (conformity index: 0.87 vs. 0.61, p = 0.00) and integral dose to OARs and normal tissue, although a greater volume of normal tissue receiving dose below 10 Gy was observed. The results were similar in HT except that the integral dose to normal tissue increased slightly. Compared directly with IMRT, HT showed better dose homogeneity and lower integral dose to rectum and bladder, but the integral dose to pelvic bones and normal tissue slightly increased. Conclusions. In postoperative WPRT of endometrial cancer, IMRT and HT result in better conformity and lower integral dose to OARs compared with 3D-CRT. The integral dose to normal tissue did not increase significantly in IMRT, although a greater volume of normal tissue is irradiated to the dose below 10 Gy. HT further improves the dose homogeneity and integral dose to rectum and bladder, at the expense of a slightly higher integral dose to pelvic bones and normal tissue.
Radiotherapy is commonly used in the treatment of endometrial cancer. Randomized trials have demonstrated that whole pelvic radiotherapy (WPRT) reduces the rate of pelvic disease recurrence in patients who have undergone hysterectomy for endometrial cancer [Citation1,Citation2]. However, conventional WPRT with three-dimensional conformal radiotherapy (3D-CRT) exposes most of the contents of the pelvis to the prescribed dose, due to the cup-shaped tissue volume produced by the pelvic floor and iliac lymph nodes [Citation3]. A significant portion of small bowel falls into the vacated space in the pelvis after hysterectomy, increasing the volume of bowel treated to high dose. This in turn increases the risk of acute and late small bowel complications, limiting the dose that can be delivered to paravaginal and nodal tissues that are at risk for recurrence. Although severe chronic toxicities (proctitis, obstruction, fistulas) are uncommon, many women treated with WPRT suffer from a variety of chronic problems including intermittent diarrhea, intolerance to certain foods, and malabsorption of vitamins, lactose and bile acids [Citation4,Citation5]. In addition, a large volume of pelvic bone marrow is also irradiated. Because most of the total body bone marrow reserve is located within the lower lumbar spine and pelvic bones, hematologic toxicity is common in gynecologic patients treated with concomitant whole pelvic radiotherapy and chemotherapy [Citation6]. Severe hematologic toxicity, although rarely life-threatening, can lead to delayed or missed chemotherapy, hospitalizations, the need for growth factors, and a limited ability to delivery combination chemotherapy [Citation6–8].
The use of IMRT is increasing in gynecological patients [Citation9]. It has been shown to be a promising approach for better sparing of adjacent critical structures for WPRT [Citation5,Citation10]. IMRT has also been reported to significantly reduce the acute and late toxicities of organs at risk (OARs) [Citation11–13]. Helical tomotherapy (HT) has recently become an attractive approach of IMRT that delivers extremely conformal dose distributions in a helical pattern. The improved dose homogeneity and better sparing of critical structures in HT compared with conventional linac-based IMRT has been reported in many cancers [Citation14–18].
Compared with 3D-CRT, IMRT and HT involve more radiation fields. As a consequence, a greater volume of normal tissue is exposed to relatively lower doses. Therefore, there has been concern about the increase of integral dose to normal tissue with IMRT and HT as a potential risk factor for the development of secondary malignancies [Citation19]. The potential increase of integral dose to normal tissue with IMRT remains controversial. Some studies have found an increase in the integral dose with IMRT [Citation20,Citation21], and others have reported a decrease [Citation22,Citation23]. However, it may not be appropriate to extrapolate any one of these conclusions to WPRT for postoperative endometrial cancer because of the different geometry of targets and OARs. To date, no published studies have performed a dosimetric evaluation of WPRT using HT for postoperative endometrial cancer. The purpose of this study was to perform a direct dosimetric comparison of 3D-CRT, IMRT and HT plans for WPRT in postoperative endometrial cancer patients, and to evaluate the integral dose to normal tissue and OARs.
Material and methods
Patient selection and simulation
Ten patients with endometrial cancer undergoing postoperative WPRT were selected for this study. For each patient, a vaginal marker was inserted to indicate the position of the vaginal apex, carefully not to distort the vagina before the simulation scan. All patients were instructed to drink 1 500 ml water one hour before simulation and treatment. Then the patients were immobilized using thermoplastic mask, and scanned from T12 vertebrate to mid-thigh, with the slice thickness of 0.5 cm. In addition, oral and i.v. contrast were administrated to all patients before the computed tomography (CT) scan. The image sets were transferred to the Pinnacle3 treatment planning system (Version 7.6, Philips Medical System, Milpitas, CA) for contouring and planning. This study was approved by the institutional review board and informed consent was obtained.
Contour of targets
The clinical target volume (CTV) was delineated according to the consensus guidelines of RTOG [Citation24]. The CTV included pelvic lymph node regions (common, internal and external iliacs), the proximal 3.0 cm of the vagina and paravaginal tissues for all the patients. For patients with cervical stromal invasion, the presacral lymph node region was also contoured to the inferior border of S2. A margin of 0.7 cm was added to the “vessels” contour in all dimensions and modified by anatomic boundaries (as clinically indicated for individual patients) to create the nodal clinical target volume, from which the pelvic bones, femoral heads, and vertebral bodies were excluded. The CTV was expanded by 1 cm to create the planning target volume (PTV).
Contour of OARs and normal tissue
The OARs contoured include the bladder, rectum, small intestine, colon and pelvic bone marrow. The superior and inferior extents of OARs were outlined on all CT slices in which portions of the PTV existed, as well as at an additional 2 cm superior and inferior to the limits of the PTV. The rectum was contoured from the rectosigmoid flexure to anus. The small intestine and colon were defined as all individual bowel loops, and contoured together as one structure referred to as the “bowel”. The normal tissue is defined as the entire irradiated volume within the skin surface minus the PTV. The pelvic bone marrow was defined and contoured according to the method described by Mell et al. [Citation25]. The external contour of all pelvic bones was delineated to define the bone marrow. The external contour was used, rather than the intramedullary space within the bones, to reduce the bias of contouring due to the CT windowing and leveling. The entire bony contour consisted of ilium, lower pelvis and lumbosacral spine. No expansion of all these OARs was made to account for the organs motion and set up error.
Treatment planning
3D-CRT and static IMRT plans were generated for each patient using Pinnacle3 planning system (Version 7.6, Philips Medical System, Milpitas, CA). 3D-CRT four-field box plans were generated using 18-MV photons. The beam aperture was shaped to the PTV in each beam's eye view, with a 0.5 cm margin in all directions to account for beam penumbra. Weights of the individual fields were optimized to maximize homogeneous dose distribution to the PTV, and minimize the dose to the OARs. Based on the findings of previous studies [Citation26,Citation27] and our pilot study, the IMRT plans using 6 MV photons were generated using nine coplanar fields. The fields were equally spaced at the interval of 40° with the starting angle of 0°. The typical dose-volume constraints of IMRT, used as input for the inverse treatment planning process, were given in .
Table I. Dose-volume constraints used in IMRT
The HT plan was created using Tomotherapy treatment planning system (Hi-Art Tomotherapy, TomoTherapy Inc., Madison, WI) for each patient. CT datasets with structures contoured in Pinnacle3 planning system were transferred to the Tomotherapy planning system using Digital Imaging and Communication in Medicine RT protocol. The optimization was guided with dose volume constraints, precedence, importance, and penalty parameters, which were set based on the results of IMRT and our pilot study. A field width of 2.5 cm was used for all plans, along with a pitch of 0.3 and a modulation factor of 3.0.
Dosimetric comparison
For the convenience of comparison, all plans were normalized to deliver 50 Gy to 95% of the PTV. The DVHs of the 3D-CRT, IMRT and HT plans were compared for the PTV coverage, OARs and normal tissue sparing, and integral dose to OARs and normal tissue. The parameters analyzed included the percentage of PTV receiving 95%, 100%, 105%, and 110% of the prescription dose (PTV95, PTV100, PTV105, and PTV110); the homogeneity index (HI) and conformity index (CI). The HI was defined as D5%/D95% (minimum dose in 5% of the PTV volume that received the most dose/minimum dose in 95% of the PTV volume that received the most dose) [Citation28]. Since not all parts of the PTV were covered by the prescribed dose, the CI was calculated as follows: CI=CF (cover factor) X SF (spill factor), where the CF was defined as the percentage of the PTV volume receiving at least the prescribed dose and the SF as the volume of the PTV receiving at least prescription dose relative to the total prescription dose volume [Citation28]. The closer the CI value is to 1, the better the dose conformity. To quantify the dose distribution of OARs and normal tissue in different dose level, the percentage volume of the OARs and normal tissue receiving a dose of 5 Gy, 10 Gy, 20 Gy, 30 Gy, 40 Gy and 50 Gy (V5, V10, V20, V30, V40 and V50) were evaluated and compared for three techniques. The mean dose and integral dose to OARs and normal tissue were also compared. The integral dose is equal to the mean dose times the volume of each structure.
Statistics
To compare the three kinds of techniques, the results of 3D-CRT were designated as the baseline and compared with those of IMRT and HT. A direct comparison of the dosimetric parameters between IMRT and HT was also performed, by designating IMRT as the baseline and compared with HT. The significance of differences was tested using a paired two-tailed Student t-test. The threshold for statistical significance was p < 0.05. All data were analyzed using Statistical Package for Social Science, version 13.0, software (SPSS, Chicago, IL).
Results
PTV coverage
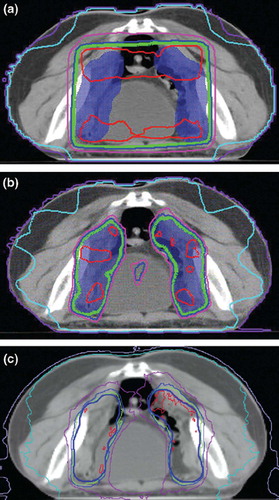
summarizes the PTV coverage for three techniques. The mean conformity index was 0.61, 0.87 and 0.87 for 3D-CRT, IMRT and HT plans, respectively. IMRT and HT significantly improve the dose conformity compared to 3D-CRT (p < 0.01). The conformity index was not significant different for HT plans compared to IMRT plans (p > 0.05). HT also improved the dose homogeneity compared with 3D-CRT and IMRT. Specifically, the average HI was 1.08, 1.10 and 1.07, the mean PTV110 was 1.1%, 7.6% and 0.6% for 3D-CRT, IMRT and HT plans, respectively. A typical axial dose distribution obtained with three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy is given in .
Table II. Summary of PTV coverage data for 3D-CRT, IMRT and HT plans
OARs and normal tissue sparing
The DVHs of OARs and normal tissue are listed in . For the rectum, the V40 and V50 were significantly lower in IMRT and HT plans. For the bladder, the percentage volume receiving dose above 30 Gy and 20 Gy was also significantly reduced with IMRT and HT, respectively. The V5 and V10 of the bowel were higher in HT plans, but the volume of bowel receiving dose above 20 Gy significantly decreased in IMRT and HT plans. For the pelvic bones, the volume percentage receiving a dose of at least 20 Gy also decreased with IMRT and HT, although the V5 and V10 were slightly increased in HT plans. The mean dose to OARs and normal tissue decreased in IMRT and HT plans except that the difference of mean dose to normal tissue between 3D-CRT and HT was not statistically significant. However, IMRT and HT significantly increased the normal tissue volume receiving the dose below 10 Gy as compared with 3D-CRT.
Table III. Summary of OARs and normal tissue dose distribution for 3D-CRT, IMRT and HT plans
When analyzing the HT plans, with the IMRT plans as the baseline, HT further decreased the volume of the rectum and bladder receiving dose above 30 Gy. But the V5 and V10 of bowel increased 5.0 and 7.0 (p < 0.01). The volume of the pelvic bones and normal tissue receiving a dose below 20 Gy also increased in the HT plans (p < 0.05).
Integral dose to OARs and normal tissue
The integral dose to OARs and normal tissue for three techniques are summarized in . Both the IMRT and HT plans resulted in lower integral dose to the OARs (5–28%, p < 0.05) compared to 3D-CRT. The integral dose to normal tissue was 7.96% lower in IMRT (p < 0.01) compared to 3D-CRT. Although the integral dose to normal tissue was slightly higher in HT plans, the difference was small between 3D-CRT and HT (4.83%, p = 0.04). A direct comparison with IMRT, HT further decreased the integral dose to rectum and bladder (p < 0.05), whereas gave rise to slightly greater integral dose to pelvic bones and normal tissue (p < 0.05).
Table IV. Integral dose to OARs and normal tissue for 3D-CRT, IMRT and HT plans
Discussion
In this study, we compared three kinds of pelvic radiotherapy treatment planning for postoperative endometrial cancer. This is the first study to provide a dosimetric evaluation of WPRT using 3D-CRT, IMRT and HT, to evaluate the integral dose to normal tissue and OARs. The benefit of IMRT and HT was manifested by the excellent conformity to PTV. Our results suggest that IMRT results in more conformal PTV coverage and better sparing of OARs than does 3D-CRT. These results are consistent with the data of previous publications [Citation10,Citation26]. The integral dose to OARs also decreased significantly in this study. Similar results were observed in HT plans. Compared directly with IMRT, HT resulted in improved dose homogeneity and lower dose to rectum and bladder, with comparable conformity in this study, which was consistent with the results of previous publications on other cancers [Citation14–18]. It might be explained by the greater degree of freedom of intensity modulation with helical pattern of dose delivery and unique binary MLC.
As expected, the volume of normal tissue receiving low dose increased in IMRT and HT. However, IMRT did not increase the integral dose to normal tissue. Aoyama et al. [Citation18] evaluated the integral dose to normal tissue of IMRT plans for prostate cancer. They found that 6 MV-IMRT resulted in 5.0% lower integral dose to normal tissue than 6MV-3DCRT. Similar results were observed in the publications of Hermanto et al. [Citation22] and Mock et al. [Citation23] for glioma and paranasal sinus carcinoma, although it is commonly believed that the large number of beamlets and monitor units used in IMRT leads to an increase in the integral dose to normal tissue [Citation19]. In contrast to a previous study [Citation18], HT slightly increased the integral dose to normal tissue in this study. But, the difference in integral dose to normal tissue between HT and 3D-CRT is less than <5%. Aoyama et al. [Citation18] found that HT improved the sparing of the rectal wall and the penile bulb compared to IMRT and 3D-CRT for localized prostate cancer. They also found that the integral dose decreased by 4% compared to 3D-CRT. Pirzkall et al. [Citation29] evaluated the effect of beam energy and number of fields on photon-based IMRT for prostate cancer. They also found that the difference of integral nontarget dose was within 5% for all plans. The small difference is likely due to the balance of greater volume of normal tissue receiving low dose and smaller volume receiving high dose. Compared with IMRT directly, the integral dose to normal tissue and pelvic bones increased in HT. The increased integral dose to normal tissue and pelvic bones might be attributable to the larger and longer target volumes exposed to more radiation beams in the helical pattern of radiation delivery. This is further supported in that the V5 of OARs and normal tissue were higher in HT (). D’Souza and Rosen [Citation30] reported that the total energy deposited in a patient is relatively independent of treatment planning parameters for deep-seated targets, the integral dose to normal tissue increases with increasing size of the anatomic region for similar tumor sizes. The mean volume ratio of normal tissue to PTV was 12.3 (range: 9.6–14.3) in our study. It is possible that the integral dose in the pelvic bones and normal tissue could be decreased in the planning process by introducing the corresponding dose volume constraints. Because this study was designed to be a comparative dosimetric evaluation of three techniques, we did not use any constraints for pelvic bones and normal tissue, and used the same dose volume constraints in IMRT and HT based on our experience and pilot study. In order to define the optimal beam configuration in IMRT, and the optimal dose-volume constraints used in IMRT and HT, plans for five patients were generated using five, seven, nine and 11 equally spaced, coplanar photon beams in the pilot study. The results demonstrated that increasing the beam number was associated with better dose conformity and sparing of organs at risk. However, no significant improvement was observed when using more than nine beams, with larger volume of normal tissue exposed to low dose. In addition, our data showed that both IMRT and HT could achieve comparable PTV coverage with the same DVH constraints. Of course, it is also possible that there might be slight differences in the results due to the different optimization algorithm used in each of the unique planning system.
Whole pelvic IMRT has been reported to reduce the rate of acute [Citation31] and chronic gastric-intestinal toxicity than conventional WPRT [Citation12]. A promising trend of lower incidence rate of acute hematologic toxicity with IMRT compared with conventional WPRT was observed in previous study [Citation13], particularly in the patients receiving chemotherapy. There are no reported clinical results of gynecologic cancer patients treated with whole pelvic helical tomotherapy. The improved homogeneity, conformity and sparing of OARs in HT is expected to further reduce the acute and late toxicities of OARs, especially for the patients requiring local boost and concurrent/sequential chemotherapy. However, greater volume of pelvic bones exposed to a dose of below 10 Gy could increase the risk of hematologic suppression [Citation25] and bone fracture [Citation32]. Some may raise concerns regarding the risk of secondary cancers in the larger volume of normal tissue irradiated to low dose. Given the life expectancy of the older patients with endometrial cancer, the risk may be small. The parallel clinical study is undergone to assess the potential clinical benefits and pitfall of the dosimetric differences.
Conclusions
In postoperative WPRT of endometrial cancer, IMRT and HT result in more conformal dose distribution and lower integral dose to OARs compared with 3D-CRT. The integral dose to normal tissue did not increase significantly in IMRT, although a greater volume of normal tissue is irradiated to the dose below 10 Gy. HT further improves the dose homogeneity and integral dose to rectum and bladder, at the expense of a slightly higher integral dose to pelvic bones and normal tissue. The clinical significance of these dosimetric differences needs to be further investigated.
Acknowledgement
The authors declare that they have no competing interest.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Henry MK, James AR, Virginia LB, Richard JZ, Nick MS, Jeffrey DB, . A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–51.
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, . Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomized trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet 2000;355 (9213):1404–11.
- Ahamad A, D’Souza W, Salehpour M, Iyer R, SL Tucker, Jhingran A, . Intensity-modulated radiation therapy after hysterectomy: Comparison with conventional treatment and sensitivity of the normal-tissue-sparing effect to margin size. Int J Radiat Oncol Biol Phys 2005;62:1117–24.
- Roeske JC, Mundt AJ, Halpern H, Sweeney P, Sutton H, Powers C, . Late rectal sequelae following definitive radiation therapy for carcinoma of the uterine cervix: A dosimetric analysis. Int J Radiat Oncol Biol Phys 1997;37:351–8.
- Perez CA, Grigsby PW, Lockett MA, Chao KS, Williamson J. Radiation therapy morbidity in carcinoma of the uterine cervix: Dosimetric and clinical correlation. Int J Radiat Oncol Biol Phys 1999;44:855–66.
- Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, . Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 1999;340:1144–53.
- Dueñas-González A, Cetina-Perez L, Lopez-Graniel C, Gonzalez-Enciso A, Gómez-Gonzalez E, Rivera-Rubi L, . Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer: A randomized Phase II study. Int J Radiat Oncol Biol Phys 2005;61:817–23.
- Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, . Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet 2001;358(9284):781–6.
- Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer 2005;104:1296–303.
- Lujan AE, Mundt AJ, Yamada SD, Rotmensch J, Roeske JC. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2003;57:516–21.
- Beriwal S. Clinical outcome with adjuvant treatment of endometrial carcinoma using intensity-modulated radiation therapy. Gynecol Oncol 2006;102:195–9.
- Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecologic patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys 2003;56:1354–60.
- Brixey CJ, Roeske JC, Lujan AE, Yamada SD, Rotmensch J, Mundt AJ. Impact of intensity-modulated radiation therapy on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;54:1388–96.
- Lee TF, Fang FM, Chao PJ, Su TJ, Wang LK, Leung SW. Dosimetric comparisons of helical tomotherapy and step-and-shoot intensity-modulated radiotherapy in nasopharyngeal carcinoma. Radiother Oncol 2008;89:89–96.
- Van Vulpen M, Field C, Raaijmakers CP, Parliament MB, Terhaard CHJ, MacKenzie MA, . Comparing step-and-shoot IMRT with dynamic helical Tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2005;62:1535–9.
- Sheng K, Molloy JA, Read PW. Intensity-modulated radiation therapy (IMRT) dosimetry of the head and neck: A comparison of treatment plans using linear accelerator-based IMRT and helical tomotherapy. Int J Radiat Oncol Biol Phys 2006;65:917–23.
- Han C, Liu A, Schultheiss TE, Pezner RD, Chen YJ, Wong JY. Dosimetric comparisons of helical tomotherapy treatment plans and step-and-shoot intensity-modulated radiosurgery treatment plans in intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 2006;65:608–16.
- Aoyama H, Westerly DC, Mackie TR, Olivera GH, Bentzen SM, Patel RR, . Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys 2006;64:962–7.
- Hall EJ, Wuu CS. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83–8.
- Thilmann C, Sroka-Perez G, Krempien R, Hoess A, Wannenmacher M, Debus J. Inversely planned intensity modulated radiotherapy of the breast including the internal mammary chain: A plan comparison study. Technol Cancer Res Treat 2004;3:69–75.
- Pirzkall A, Carol M, Lohr F, Höss A, Wannenmacher M, Debus J. Comparison of intensity-modulated radiotherapy with conventional conformal radiotherapy for complex-shaped tumors. Int J Radiat Oncol Biol Phys 2000;48:1371–80.
- Hermanto U, Frija EK, Lii MJ, Chang EL, Mahajan A, Woo SY. Intensity-modulated radiotherapy (IMRT) and conventional three-dimensional conformal radiotherapy for high-grade gliomas: Does IMRT increase the integral dose to normal tissue? Int J Radiat Oncol Biol Phys 2007;67:1135–44.
- Mock U, Georg D, Bogner J, Auberger T, Potter R. Treatment planning comparison of conventional, 3D conformal, and intensity-modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys 2004;58:147–54.
- Small Jr W, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, . Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:428–34.
- Mell LK, Kochanski JD, Roeske JC, Haslam JJ, Mehta N, Yamada SD, . Dosimetric predictors of acute hemato-logic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:1356–65.
- Roeske JC, Lujan AE, Rotmensch J, Waggoner SE, Yamada D, Mundt AJ. Intensity-modulated whole pelvis radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2000;48:1613–21.
- Mell LK, Tiryaki H, Ahn KH, Mundt AJ, Roeske JC, Aydogan B. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys 2008;71:1504–10.
- Weiss E, Wijesooriya K, Ramakrishnan V, Keall PJ. Comparison of intensity-modulated radiotherapy planning based on manual and automatically generated contours using deformable image registration in four-dimensional computed tomography of lung cancer patients. Int J Radiat Oncol Biol Phys 2008;70:572–81.
- Pirzkall A, Carol MP, Pickett B, Xia P, Roach M, Verhey LJ. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. Int J Radiat Oncol Biol Phys 2002;53:434–42.
- D’Souza WD, Rosen II. Nontumor integral dose variation in conventional radiotherapy treatment planning. Med Phys 2003;30:2065–71.
- Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, Roeske JC. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;52:1330–7.
- Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA 2005;294:2587–93.