Abstract
Aim. To determine the additional value of FDG-PET-CT as compared to conventional staging (CS) in high-risk breast cancer patients. Patients and methods. Thirty-one high-risk breast cancer patients, 14 of whom had recurrent breast cancer, were included in this study, which took place between June 2005 and March 2008. None of the patients had clinical signs of distant metastases. FDG-PET-CT scanning was added to CS, which consisted of a chest x-ray, liver ultrasonography or CT, and bone scintigraphy. Median follow-up was 17 months (6–41 months). FDG-PET-CT was considered to have additional value to CS if it led to a change in treatment plan or if it made additional examinations to confirm or deny findings on CS unnecessary. Results. FDG-PET-CT was considered to have additional value to CS in 13 patients (42% [95% CI: 23–61]). In five patients (16% [95% CI: 1–31]), FDG-PET-CT led to a change in treatment plan by identifying nodal metastases in the internal mammary chain (IMC; N = 3) or in the mediastinum (N = 2). In nine patients (29% [95% CI: 11–47]), FDG-PET-CT would have prevented the need for additional examinations; in seven of these nine patients, distant metastases were suggested in bone or liver on CS, but these did not show FDG uptake. Conclusions. FDG-PET-CT was found to have additional value to CS in 42% of the patients. To optimize cost-effectiveness, the main challenge now is to improve the selection of patients in whom FDG-PET-CT has additional value to CS.
Adequate staging is important for the optimal choice of treatment in high-risk breast cancer patients. Although current guidelines [Citation1] recommend the use of chest x-ray, liver ultrasonography (US) or CT, and bone scintigraphy, these imaging modalities have been shown to be less sensitive and specific than 18Fluoro-Deoxy-Glucose Positron Emission Tomography (FDG-PET) [Citation2–12]. In a study of 60 breast cancer patients, Dose et al. [Citation11] found a sensitivity of 86% for FDG-PET versus 57% for conventional staging; for specificity, these figures were 90% and 81%, respectively. More recently, Fuster et al. [Citation10] found even better values for FDG-PET-CT. They too analyzed 60 breast cancer patients and found a sensitivity of 100% for FDG-PET-CT versus 60% for conventional imaging; for specificity, these values were 98% versus 83%.
Many studies have focused on the additional value of FDG-PET(-CT) in altering treatment management. Up to 42% of patients have been reported to be up-or downstaged with FDG-PET(-CT) [Citation8,Citation10,Citation13], with consequences for treatment in 10–30% of the patients [Citation8,Citation9,Citation13]. FDG-PET(-CT) is also much more convenient with respect to logistics. It requires only one examination, whilst conventional staging takes at least three examinations (i.e. bone scintigraphy, chest x-ray, and ultrasonography or CT of the liver). Furthermore, due to the relatively poor specificity of conventional staging, additional diagnostic procedures are often needed to deny or confirm metastases, causing distress and delay of the start of treatment [Citation14]. We know of only one study that compared the number of required additional examinations between FDG-PET and conventional imaging [Citation4]. It showed that conventional imaging generated more (17%) additional tests than FDG-PET (5%). For FDG-PET-CT, this difference may even be larger [Citation5].
In summary, the superior sensitivity and specificity of FDG-PET(-CT) over conventional staging is expected to have considerable additional value, not only because it leads to a change in treatment plan in up to 30% of patients, but also because it can reduce the need for additional diagnostic exams in a substantial proportion of patients. However, FDG-PET-(CT) is obviously much more expensive than conventional staging. The cost-effectiveness of staging with FDG-PET-CT might be increased if we could develop a prediction model to calculate the probability of FDG-PET-CT having additional value to conventional staging for an individual patient. Such a prediction model would call for FDG-PET-CT staging only in patients with a high probability of gaining additional value from it. The first aim of this prospective pilot study was, therefore, to evaluate the additional value that FDG-PET-CT might have over conventional staging in high-risk breast cancer patients. As a secondary aim, we explored whether patient characteristics could be identified that might be correlated with the probability of FDG-PET-CT having this additional value.
Patients and methods
Patients
Between June 2005 and March 2008, 31 patients were enrolled in this prospective study. All had a minimum follow-up of six months calculated from the date of the FDG-PET-CT scan. Patients were eligible if they had cT1-2N0-2 invasive breast cancer with any of the following poor prognostic characteristics: grade III, tumor diameter > 3 cm, clinically positive axillary nodes, or age younger than 40 years (N = 9); a locally advanced breast cancer (cT3N1-2, any cT4, and any N3) (N = 8); or a loco-regional recurrence without clinical evidence of distant metastases (N = 14) (). All patients had an invasive ductal carcinoma; none had diabetes.
Table I. Patients’ charateristics
The study was approved by the Medical Ethics Committee of MAASTRO clinic, according to Dutch law and regulations. Written informed consent was given by all patients.
Staging
Conventional staging was performed in all patients according to Dutch guidelines (www.oncoline.nl), and consisted of chest x-ray, ultrasonography (US) or CT of the liver, and bone scintigraphy. In addition, an FDG-PET-CT scan was performed. Twenty-one patients were scanned using a Gemini® PET-CT (Philips Medical Systems) scanner with time-of-flight (TOF) capability, together with a 64-slice Brilliance CT scanner. This scanner has a transverse and an axial Field of View (FOV) of 57.6 and 18 cm, respectively. The spatial resolution is around 5 mm. Ten patients were scanned using a Siemens Biograph™ (SOMATOM® Sensation 16 with an ECAT ACCEL PET scanner and 16-slice CT, Siemens) with a FOV of 58.2 cm (transverse), an axial FOV of 16.2 cm, and a spatial resolution of 6–7 mm.
Patients fasted for at least six hours before the examination. In all patients blood glucose was measured to ensure that it was below 10 mmol/l. F18-FDG (MDS Nordion, Liège, Belgium) was injected intravenously, followed by physiologic saline (10 ml). The injected total activity of FDG depended on the weight of the patient: (weight (kg) *4 + 20) MBq. After a resting period of 45 minutes (time needed for uptake of FDG), PET and CT images were acquired from the head to the upper legs. A low-dose CT-scan was performed without intravenous contrast and used for attenuation correction of the PET emission images. The PET images were acquired in five-minute bed positions. The complete PET data set was reconstructed iteratively with a reconstruction increment of 5 mm to provide isotropic voxels.
Treatment
The diagnostic results were discussed in the multi-disciplinary tumor board, and decisions on surgery, radiotherapy, and/or systemic treatment were made according to Dutch guidelines (www.oncoline.nl). If no distant metastases were found, loco-regional treatment was given, consisting of breast-conserving or ablative surgery in combination with a sentinel node procedure and/or axillary lymph node dissection. Post-operative radiotherapy was always applied in cases of breast-conserving surgery. Elective irradiation of the supraclavicular nodes (and of the chest wall in cases of mastectomy) was performed in patients with four or more positive axillary nodes, in patients with pT3N1 disease, in patients with T4 disease, and in all cN1-2 patients treated with neoadjuvant chemotherapy. Elective irradiation of the axilla and IMC was not performed, but, in cases of regional metastases to the internal mammary chain or supraclavicular nodes, these were treated with radiotherapy 50–66 Gy for 5–6.5 weeks. In cases of distant metastases, radical loco-regional treatment was omitted and patients were treated with palliative systemic treatment, with or without palliative radiotherapy on indication.
Analysis
Diagnostic data were re-evaluated to determine whether replacing conventional imaging with FDG-PET-CT yielded additional value, i.e., either because it eliminated the need for additional examinations (e.g. MRI) of lesions seen on conventional imaging, or because it led to a change in treatment plan. First we determined whether additional examinations would have been performed if we had considered the conventional imaging or the FDG-PET-CT data separately. Based on these data, we determined for each patient whether FDG-PET-CT reduced or increased the need for additional examinations compared to conventional imaging. Second, we determined the treatment plan according to whether only the conventional imaging or only the FDG-PET-CT would be used. Based on these data, we determined in which patients FDG-PET-CT led to a change in treatment plan. Finally, we determined for each patient whether replacing conventional imaging with FDG-PET-CT had additional value. This was done by scoring (1) whether additional examinations were deemed unnecessary with FDG-PET-CT and/or (2) whether the treatment plan had changed. We also determined whether we could identify patients in whom conventional staging had additional value to FDG-PET-CT staging.
In addition, an exploratory data analysis was performed to identify patient characteristics that might predict whether FDG-PET-CT has additional value to conventional staging. Due to the small number of patients included in this study, only descriptive results are presented for these analyses; statistical tests were not performed.
Results
Overall findings
In all patients the primary tumor or recurrence showed FDG uptake. Metastases to axillary nodes were found in 17 patients, to the periclavicular nodes in six patients, to the internal mammary chain nodes (IMCN) in four patients, and to the mediastinal nodes in three patients. The axillary and periclavicular metastases were detected both with conventional staging and on FDG-PET-CT in all but two patients. Metastases to the IMCN and mediastinal nodes were only detected by FDG-PET-CT ().
Figure 1. Overview of the most important lesions found on staging. CS stands for conventional staging; IMC for internal mammary chain; FNA for fine needle aspiration; DM for distant metastases.
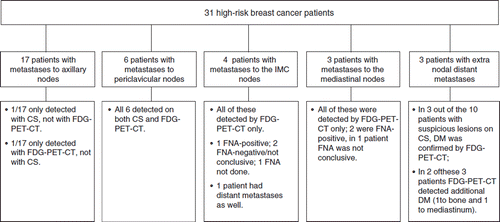
Extranodal distant metastases were found in three patients. Distant metastases were, however, suspected on conventional staging in ten patients; in only three of these patients were distant metastases confirmed by FDG-PET-CT. Of the other seven patients with negative FDG-PET-CT, six were still without evidence of disease at 8–41 months, suggesting that these lesions were, in fact, false-positive. One of the seven patients with a presumed false-positive hotspot on bone scintigraphy developed liver metastases after six months, without evidence of bone metastases at that time-point.
Additional value of FDG-PET-CT
In 18 of the 31 patients (58%, [95% CI: 39–77]), FDG-PET-CT was considered to be of no additional value (). In 15 of these 18 patients, the FDG-PET-CT findings were equal to those of conventional staging. In two of the three patients with discordant findings, the discrepancy concerned axillary staging ().
Figure 2. Overview of patients who were and were not considered to have additional value of the FDG-PET-CT. CS stands for conventional staging; IMCN for internal mammary chain nodes; FNA for fine needle aspiration; DM for distant metastases; NED for no evidence of disease; ALND for axillary lymph node dissection; RT for radiotherapy.
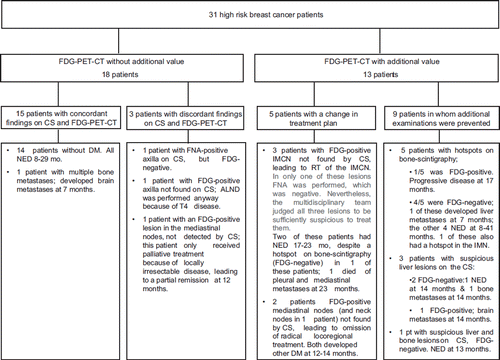
In 13 of the 31 patients (42%, 95% CI: [23–61]), FDG-PET-CT was considered to be of additional value to conventional staging (). In nine of these 13, conventional staging alone would have led to additional examinations because of suspicious lesions in bone (five patients) or liver (three patients), or a combination of bone and liver (one patient). In only two of these nine patients were distant metastases confirmed on FDG-PET-CT; in two other patients, the FDG-PET-CT was negative, but distant metastases developed elsewhere. In five patients, FDG-PET-CT had additional value because it led to a change in treatment plan. In three patients, it concerned the detection of nodes in the IMC, leading to extension of the radiation portals. In only one of these three patients was FNA performed, and this was negative. In spite of the negative FNA, the multidisciplinary tumor board decided to treat the IMC due to doubt about the representativeness of the FNA. In the other two patients, the multidisciplinary tumor board considered FNA not to be necessary due to pathology-proven extensive loco-regional extension in other nodes as well. In the two other patients with a changed treatment plan, aggressive loco-regional treatment was not given due to the detection of distant metastases in the mediastinal nodes (and neck nodes in one patient). Although these lesions were not proven by biopsy, the development of other distant metastases at 12 and 14 months respectively confirmed our hypothesis that the FDG-PET-CT lesions indeed concerned distant metastases.
No patients were found in whom conventional staging had additional value to FDG-PET-CT.
Patient characteristics associated with additional value of FDG-PET-CT
In 50% of the patients with localization of the tumor in the medial part of the breast or with supraclavicular lymph node metastases, FDG-PET-CT had additional value; this figure was only 37% in patients without medial or supraclavicular tumor involvement. For patients with locally advanced or recurrent breast cancer, FDG-PET-CT was of additional value in 50% of the cases, whereas this figure was only 22% in patients with early stage high-risk breast cancer. For patients with or without a positive axilla on palpation or ultrasonography, these figures were also 50% and 22%, respectively. The mean age of patients for whom FDG-PET-CT was of additional value was 53 years (± 11 year, 1 SD) versus 45 years (± 12 year, 1 SD) for patients in whom conventional staging was sufficient. The mean tumor size was similar for patients for whom FDG-PET-CT was of additional value and for patients for whom it was not, namely, 35 mm (± 29 mm, 1 SD) and 35 mm (± 35 mm, 1 SD), respectively.
Discussion
We found that FDG-PET-CT had additional value to conventional staging in 42% [95% CI: 23–61] of the patients; in 13% [95% CI: 1–31] of the patients, FDG-PET-CT led to a change in treatment plan. The most important finding inducing a change in treatment plan was the detection of metastases to the internal mammary chain nodes (three patients) and to the mediastinum (two patients). Apart from changing the treatment plan, we found that suspicious bone and liver metastases found on conventional imaging would have required additional examinations in nine patients (29% [95% CI: 11–47]); in seven of these patients, the FDG-PET-CT was negative. In other words, in seven patients (23% [95% CI: 7–39]) an unnecessary period of distress and anxiety would have been avoided if FDG-PET-CT had been used instead of conventional staging.
Findings on PET-CT compared with conventional staging
Several studies have reported that FDG-PET-CT is very useful, especially with respect to the detection of extra-axillary nodal metastases [Citation4,Citation6,Citation7,Citation9,Citation13,Citation15,Citation16]. The group in Seattle [Citation6,Citation13,Citation16] found as many as 25–40% metastases to the internal mammary chain or mediastinum in patients staged for recurrent or metastatic breast cancer. In patients with less advanced stages, lower figures were found, i.e., only 7% in patients prospectively staged for primary breast cancer, stages I–III [Citation15]. These findings correspond quite well with our findings. We studied patients with primary high-risk breast cancer and found additional extra-axillary nodes in the internal mammary chain and the mediastinal nodes in 22% of patients (four and three patients, respectively; ). All supraclavicular nodes had already been detected by conventional staging.
In our study, distant extranodal metastases were only detected and/or suspected in the bones and liver. Bone scintigraphy appeared to have a high false-positive rate: only two of the five suspicious lesions were confirmed by FDG-PET-CT. In total, we found thus three false-positive lesions on the bone scan in 31 patients, which corresponds quite well to the findings of Fuster et al., who found a false-positive rate of bone metastases in 7 of 60 patients [Citation10]. From a recent meta-analysis, Shie et al. [Citation17] indeed concluded that the sensitivity of FDG-PET was similar to that of bone scintigraphy, but that FDG-PET had a much higher specificity.
Three of the four liver lesions found on conventional staging were considered to be false-positive since they did not show FDG-uptake; after 13–14 months, liver metastases were still not detected. Two of these lesions were found on CT and one on ultra-sound. This is somewhat surprising since others found a quite high accuracy of spiral CT (89.2%) [Citation18].
One limitation of our study may be that pathology was not always confirmed. However, it has already been shown that the sensitivity and specificity of FDG-PET(-CT) are much better than those of conventional staging [Citation4,Citation5,Citation10–12]. Therefore, we decided to rely on the FDG-PET-CT data when pathology confirmation was too cumbersome to obtain since this strategy would also be followed when clinically implemented. Nevertheless, in 24 of the 31 patients we did have some kind of validation: (1) concordant findings between FDG-PET-CT and conventional staging (N = 14); (2) pathology confirmation (N = 4); or (3) prolonged follow-up (N = 5–7). Although the latter method does not take into account the potential for cCR and pCR rate with adjuvant chemotherapy or the potential for prolonged suppression with adjuvant endocrine treatment, it has been used in other studies [e.g. 10,13] as a tool to distinguish between true- and false-positive lesions. In our study, seven patients had suspicious liver and/or bone lesions on conventional imaging that were FDG-negative. These were thus considered to be free of distant metastases; five of them were, indeed, without evidence of disease after 8–41 months of follow-up, and two other patients developed distant metastases at other sites after 14 months.
Additional value of FDG-PET-CT
Due to the superior sensitivity and specificity ascribed to FDG-PET(-CT) compared with conventional staging [Citation4,Citation5,Citation10–12], several authors have tried to quantify the consequences for clinical practice by calculating the percentage of patients in whom FDG-PET(-CT) is considered to induce a change in treatment plan. Most studies have concerned patients with advanced, recurrent, or metastatic breast cancer and have found changes in treatment plan in about 30% of them [Citation6,Citation8,Citation13,Citation19]. Groheux et al. [Citation9] analyzed the data of stage II and III patients and found a somewhat lower figure, 13%, which corresponds quite closely to the 16% change in treatment plan in our study.
Apart from inducing a change in treatment plan, however, another important advantage of FDG-PET(-CT) is that the number of additional examinations required to confirm or deny lesions found on staging may be reduced considerably. Gerber et al. [Citation14] found that conventional imaging called for further examinations in 12% of patients. Port et al. [Citation4] also observed that FDG-PET made considerably fewer additional examinations necessary than conventional imaging, although the number was somewhat lower than in our study: 17% for conventional staging versus 5% for FDG-PET, whereas we found that suspicious lesions on conventional staging would have called for additional examinations in 9 of the 31 patients (29%). We may have made an overestimation since, in our study, FDG-PET-CT was already used for clinical decision-making. That is, whereas in the absence of FDG-PET-CT imaging additional examinations, like MRI or CT-thorax, would have been carried out to confirm or deny lesions found on conventional imaging, these extra examinations were not actually carried out in this study when the FDG-PET-CT was considered to be conclusive. Therefore, we could not really determine the number of additional examinations associated with conventional imaging. Instead, we had to make an estimation of what would have happened if FDG-PET-CT had not been available.
The reduction in additional examinations associated with FDG-PET-CT staging not only decreases the likelihood of delaying the start of treatment and, thereby, the period in which the patient experiences severe distress due to uncertainty, but it is also likely to improve cost-effectiveness. Nevertheless, further studies are required to investigate whether reducing the need for additional testing cancels out the high costs of FDG-PET(-CT) scanning. Another step towards increasing the cost-effectiveness of FDG-PET(-CT) scanning may be to improve the selection of patients for whom FDG-PET(-CT) scanning has a high likelihood of having additional value.
Additional value of FDG-PET-CT related to patient characteristics
Although we found an additional value of FDG-PET-CT in 42% of our patients, conventional staging would have been sufficient in 58% of them. In this pilot study, we found some indications that patient characteristics, such as the localization of the tumor, age, nodal status, and/or stage, may contribute to the likelihood of FDG-PET-CT having additional value. As with our study, most of the past studies examining the value of FDG-PET(-CT) have involved too few patients to allow for an adequate statistical analysis to find a relation between patient characteristics and findings on FDG-PET-CT. This has led to conflicting findings. Tumor size and location were not found to be significant factors in the study by Eubank et al. [Citation6], whereas Bellon et al. [Citation16] did find that a larger tumor size and inflammatory disease were associated with an increased incidence of hotspots in the internal mammary chain. Tran et al. [Citation20] studied 141 breast cancer patients and found that inner quadrant tumors had a sixfold greater frequency of isolated extra-axillary metastases than outer quadrant tumors. These data suggest that there is a trend for certain patient characteristics, like tumor size and location, to correlate with the likelihood of FDG-PET-CT having additional value. However, obviously much larger studies are required to investigate this issue in more detail.
Conclusion
In conclusion, in this small pilot study, FDG-PET-CT had additional value to conventional staging in 42% of the patients. The main challenge now is to identify which patients are expected to benefit most from this expensive staging procedure. We therefore plan to extend this study to a much larger group and to develop a model that will allow us to predict the probability that replacing conventional staging with FDG-PET-CT will yield additional value for a given individual patient.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Pestalozzi B, Castiglione M, on behalf of the ESMO guidelines working group. Primary breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008;19(Suppl 2):ii7–ii10.
- Hoeven van der JJM, Krak NC, Hoekstra OS, Comans EF, Boom RP, van Geldere D, . 18F-2-Fluoro-2-Deoxy-D-Glucose positron emission tomography in staging of locally advanced breast cancer. J Clin Oncol 2004;22:1253–9.
- Oost van FJ, Hoeven van der JJM, Hoekstra OS, Voogd AC, Coebergh JW, van de Poll-Franse LV. Staging in patients with locoregionally recurrent breast cancer: Current practice and prospects for positron emission tomography. Eur J Cancer 2004;40:1545–53.
- Port ES, Yeung H, Gonen M, Liberman L, Caravelli J, Borgen P, . FDG-PET scanning affects surgical management in selected patients with high-risk operable breast carcinoma. Ann Surg Oncol 2006;13:677–84.
- Mahner S, Schirmacher S, Brenner W, Jenicke L, Habermann CR, Avril N, . Comparison between FDG-PET, conventional imaging and computed tomography for staging of breast cancer. Ann Oncol 2008;19:1249–54.
- Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, . 18Flurorodeoxyglucose positron emission tomography to detect mediastinal mammary or internal mammary metastases in breast cancer. J Clin Oncol 2001;19:3516–23.
- Danforth DN, Aloj L, Carrasquillo JA, Bacharach SL. Chow C, Zujewski J, . The role of 18F-FDG-PET in the local-regional evaluation of women with breast cancer. Breast Cancer Res Treat 2002;75:135–46.
- Dizendorf EV, Baumert BB, Schulthess von GK, Lütolf UM, Steinert HC. Impact of whole-body 18F-FDG PET on staging and managing patients for radiation therapy. J Nucl Med 2003;44:24–9.
- Groheux D, Moretti J-L, Baillet G, Espie M, Giacchetti S, Hindie E, . Effect of 18F-FDG PET-CT imaging in patients with clinical stage II and III breast cancer. Int J Radiat Oncol Biol Phys 2008;71:1–10.
- Fuster D, Duch J, Paredes P, Velasco M, Muñoz M, Santamaría G, . Preoperative staging of large primary breast cancer with [18F]Fluorodeoxyglucose positron emission tomography-computed tomography compared with conventional imaging procedures. J Clin Oncol 2008;26:4746–51.
- Dose J, Bleckmann C, Bachmann S, Velasco M, Muñoz M, Santamaría G, . Comparison of FDG-PET and “conventional diagnostic procedures” for the detection of distant metastases in breast cancer patients. Nucl Med Comm 2002;23:857–64.
- Isasi CR, Moadel RM, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast recurrence and metastases. Breast Cancer Res Treat 2005;90:105–12.
- Eubank WB, Mankoff D, Bhattacharya M, Gralow J, Linden H, Ellis G, . Impact of FDG-PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. Am J Rontgenol 2004;183:479–86.
- Gerber B, Seitz E, Muller H, Krause A, Reimer T, Kundt G, . Peri-operative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat 2003;82:29–37.
- Cermik TF, Mavi A, Basu S, Alavi A. Impact of FDG-PET on pre-operative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging 2008;35:475–83.
- Bellon JR, Livingston RB, Eubank WB, Gralow JR, Ellis GK, Dunnwald LK, . Evaluation of the internal mammary nodes by FDG-PET in locally advanced breast cancer. Am J Clin Oncol 2004;27:407–10.
- Shie P, Cardareli R, Brandon D, Erdman W, Abdulrahim N. Meta-analysis: Comparison of FDG-PET and bone scintigraphy in the detection of bone metastases in patients with breast cancer. Clin Nucl Med 2008;33:97–101.
- Dietrich CF, Kratzer W, Strobe D, Danse E, Fessl R, Bunk A, . Assessment of metastatic liver disease in patients with extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol 2006;12:1699–705.
- Klaeser B, Wiederkehr O, Koeberle D, Mueller A, Bubeck B, Thuerlimann B. Therapeutic impact of FDG-PET in the pre-and post-operative staging of patients with clinically intermediate or high-risk breast cancer. Ann Oncol 2007;18:1329–34.
- Tran A, Pio BS, Khatibi B, Czernin J, Phelps ME, Silverman DHS. FDG PET for staging breast cancer in patients with inner-quadrant versus outer-quadrant tumors. Comparison with longterm clinical outcome. J Nucl Med 2005;46:1455–9.