Abstract
Background. The risk of cardiovascular toxicities is a serious concern with the increased application of angiogenesis inhibitors in current cancer therapy. Arterial thromboembolic events (ATE) were associated with bevacizumab, an antibody against vascular endothelial growth factor. To determine the risk of ATE including cardiac ischemia and stroke, a systematic review and meta-analysis of published randomized controlled trials (RCTs) was performed. Methods. We searched the databases of PubMed, Web of Science, and American Society of Clinical Oncology conferences to identify relevant clinical trials up to May, 2009. Eligible studies included prospective RCTs in which bevacizumab was compared to a control concurrently in combination with standard anti-neoplastic therapy. Summary incidence rates, relative risks (RRs), and 95% confidence intervals (CIs) were calculated using random-effects or fixed-effects models. Results. A total of 12 617 patients with a variety of advanced solid tumors from 20 RCTs were included for analysis. The incidences of all-grade and high-grade ATE in patients receiving bevacizumab were 3.3% (95% CI, 2.0–5.6%) and 2.0% (95% CI, 1.7–2.5) respectively. Patients treated with bevacizumab had a significantly increased risk of ATE with an RR of 1.44 (95% CI, 1.08–1.91; p=0.013) compared with controls. The risk similarly increased for bevacizumab at 2.5 and 5 mg/kg/week; in addition, significantly increased risks were observed in patients with renal cell cancer (RR, 3.72, 95% CI, 1.15–12.04; p=0.029) and colorectal cancer (RR, 1.89, 95% CI, 1.28–2.80, p=0.001). Notably, the risk of high-grade cardiac ischemia with bevacizumab was significantly higher than controls with an RR of 2.14 (95% CI, 1.12–4.08, p=0.021); however, the risk of ischemic stroke with bevacizumab was not significantly different from controls (RR, 1.37, 95% CI, 0.67–2.79, p=0.39). Discussion. Treatment with bevacizumab may significantly increase the risk of cardiac ischemic events in cancer patients.
Angiogenesis plays important role in tumor progression and metastasis, and is a focal topic in the field of cancer research [Citation1–3]. This process is mainly driven by vascular endothelial growth factor (VEGF), and hence to disrupt its signaling has become a major approach in current cancer therapeutics [Citation3,Citation4]. Bevacizumab (Avastin, Genentech Inc, South San Franscisco), a recombinant humanized monoclonal antibody against VEGF, has been approved by the Food and Drug administration of USA for the treatment of many advanced solid tumors including colorectal cancer (CRC), non-small cell lung cancer (NSCLC), breast cancer, glioblastoma, and recently renal cell cancer (RCC) [Citation5]. Currently, the use of bevacizumab in other advanced cancers as well as neoadjuvant and adjuvant therapy in patients with earlier stage diseases is also undergoing extensive evaluation [Citation6–9].
Bevacizumab treatment has been associated with the occurrence of arterial thrombotic events (ATE) [Citation10]. In the pivotal placebo-controlled phase III trial for metastatic CRC by Hurwitz and colleagues, 20 of 393 patients treated with bevacizumab developed ATE as compared to five of 397 patients in controls [Citation11]. In 2007, a pooled analysis of five randomized controlled trials (RCTs) that included a total of 1 745 patients with metastatic CRC, breast, NSCLC showed that increased risk of ATE was found in the patients treated with bevacizumab in combination with chemotherapy compared to chemotherapy alone (risk ratio, 2.0; 95% CI, 1.05–3.75, p=0.031) [Citation12]. The incidence of ATE events was 3.8% in bevacizumab group as compared to 1.7% in chemotherapy alone group.
However, this study is limited by a small number of RCTs (only five trials) included for analysis. The overall risk of ATE remains unclear for general cancer patients with a variety of tumor types. Also, the relationship between bevacizumab dose and the risk of ATE needs to be defined. Because ATE includes the involvement of several different arteries such as such as coronary artery and cerebral artery, questions remain regarding the effect of bevacizumab on the risk of specific type of ATE such as cardiac ischemia or stroke. In order to understand these issues, we performed a systematic review and meta-analysis of published RCTs to determine the impact of bevacizumab on the occurrence of ATE in cancer patients.
Methods
Data source
We performed a comprehensive search of citations from PubMed between January, 1966, and May, 2009, using the keywords “bevacizumab”, “avastin”, and “cancer”. The search was limited to randomized clinical trials. The search strategy also used the text terms “arterial thrombosis”, “arterial thromboembolic events”, “angiogenesis”, “thrombosis” and “vascular endothelial growth factor” to identify relevant information. Abstracts and virtual meeting presentations from the American Society of Clinical Oncology conferences held between January, 2000, and May, 2009, were also searched to identify relevant RCTs. An independent search using the citation database Web of Science was done to ensure that all relevant clinical trials were included in the meta-analysis.
We also reviewed FDA submission documents, the updated manufacturer’s package insert. The reference lists of identified articles were examined for additional publications. We reviewed each publication; when duplicate publications were identified, only the most recent or complete report of clinical trials was included. The safety data from the manufacturer’s updated package insert of bevacizumab was also reviewed to identify relevant information, and considered to be the most recent for our analysis. Efforts were also made to contact the investigators and the manufacturer of bevacizumab when relevant data was not clear.
Study selection
The primary goal of this study was to determine whether bevacizumab contributes to the development of ATE in cancer patients. Therefore, we selected only those RCTs that directly compared patients with cancer treated with and without bevacizumab. Phase I and single-arm phase II trials were excluded due to their lack of control groups. Specifically, clinical trials that met the following criteria were included in the meta-analysis: prospective phase II and III RCTs in patients with cancer; random assignment of participants to bevacizumab treatment or control (placebo or best supportive care) in addition to concurrent chemotherapy and/or biological agent; and available data including event or incidence of arterial thrombosis and sample size. Quality was assessed using criteria including adequate blinding of randomization, completeness of follow-up, and objectivity of outcome measurements as described previously [Citation13].
Data extraction and clinical endpoints
We extracted details about the number of patients, type of cancer being treated, treatment information, grading criteria for adverse events, results, follow-up, and funding or support from the included studies. Data regarding the occurrence of ATE was obtained from the safety profile of each study. Two authors (VR and SW) extracted the data independently, and any discrepancies between the authors were resolved by consensus. As summarized in , ATE in these studies was assessed and recorded according to the National Cancer Institute’s common terminology criteria for adverse events (version 1, 2 or 3), which has been widely used in cancer clinical trials [Citation14,Citation15]. There is minor variation among these versions in grading ATE. It was graded from 1 to 4 in version 1, and from 1 to 5 in versions 2 or 3; for the study, we simply separated ATE into all grades and high grades (grade 3 and above) for our analysis. Most studies only reported high grade events.
Table I. National Cancer Institute’s common terminology criteria versions 1-3 for arterial thromboembolic events.
Statistical analysis
Version 2 of the Comprehensive Meta-Analysis program (Biostat, Englewood, NJ, USA) was used for statistical analysis. We calculated the incidence by using the number of patients with ATE and total number of patients receiving bevacizumab. The proportion of the patients with ATE and exact 95% CI were derived from each study. To calculate relative risk (RR), patients assigned to bevacizumab were compared only with those assigned to the control group in the same clinical trial. We explored a dose–effect relationship by further dividing bevacizumab therapy into low dose (5 or 7 · 5 mg/kg per dose per schedule, which is equivalent to 2 · 5 mg/kg per week) and high dose (10 or 15 mg/kg per dose per schedule, which is equivalent to 5 mg/kg per week). The low-dose and high-dose designation was arbitrary. Our previous studies have shown the dose-independent risk of venous thromboembolism, dose-dependent risk of hypertension or gastrointestinal perforation with bevacizumab at these dose levels [Citation16–18].
For the meta-analysis, we used a fixed-effects (weighted with inverse variance) or random-effects model based on the heterogeneity of included studies [Citation19]. For each meta-analysis, the Cochran’s Q statistic and I 2 statistics were first calculated to assess the heterogeneity among the proportions of the included trials. If the p-value was less than 0.10, the assumption of homogeneity was deemed invalid, and the random-effects model was reported after exploring the causes of heterogeneity [Citation20]. When results of the two models were substantially different, the random-effects model was presented. Otherwise, the fixed-effects model was reported.
Results
Search results and study quality
Our search yielded 132 potentially relevant clinical studies. After excluding ineligible studies, we selected 20 RCTs, including six phase II and 14 phase III studies for the purpose of analysis (see for selection process). Patients were enrolled according to pre-specified eligibility criteria for each trial. Randomized treatment allocation sequences were generated in all trials. Six trials were double-blinded and placebo controlled [Citation7,Citation8,Citation21–24]; five other trials had placebo as controls [Citation25–29]; the rest of the trials had active controls [Citation6,Citation30–37]. The data for three studies [Citation11,Citation28,Citation37] were obtained from the published pooled analysis study [Citation12].
ATE was assessed and recorded according to NCICTC versions 1, 2 or 3. Version 1 was used only in two trials [Citation27,Citation28], version 2 was used in eight trials [Citation11,Citation29–31,Citation34–37]; version 3 was used in six trials [Citation6,Citation21–23,Citation26,Citation32]; the rest of the trials did not specify. Follow-up time was not specified in three trials [Citation23,Citation32,Citation33]. The quality of all the trials was acceptable.
Publication bias
We used the Begg’s and Egger’s tests to determine the presence of publication bias regarding our primary endpoint (relative risk of ATE). The two-tailed p-values were 0.92 and 0.11 for Begg’s and Egger’s tests respectively. Thus, no publication bias was detected.
Patients
A total of 12 617 patients from 20 RCTs were included for analysis. The characteristics of patients and studies are listed in . The baseline Eastern Cooperative Oncology Group (ECOG) performance status for most patients was between 0 and 1. Patients were required to have adequate hepatic, renal, and hematologic function. If patients had significant cardiovascular disease, peripheral vascular disease, uncontrolled HTN, serious non-healing wounds, major surgery within previous 28 days, pre-existing bleeding diathesis, brain metastasis, regular use of ASA (>325 mg/day) or non-steroidal anti-inflammatory drugs, or were pregnant, breast feeding, or were taking oral or parental anticoagulants with the exception of prophylactic anticoagulants to maintain patency of vascular device access, they were excluded from the study. Underlying malignancies included CRC (seven studies), NSCLC (four studies), breast cancer (three studies), pancreatic cancer (two studies), RCC (three studies) and malignant mesothelioma (one study). In all trials, patients were randomly assigned to either a control or bevacizumab group, with five three arm studies each having two bevacizumab treatment groups, in which patients received different doses of bevacizumab or combination with chemotherapeutic agents.
Table II. Characteristics of randomized controlled trials included in the meta-analysis.
Incidences of ATE
A total of 1 853 patients receiving bevacizumab from eight RCTs had data for all-grade ATE for analysis (). Using a random-effects model, the summary incidence of all-grade ATE was 3.3% (95% CI, 1.28–3.40).
Table III. Incidence and relative risk (RR) of all-grade arterial thromboembolic events (ATE) with bevacizumab according to tumor types.
A total of 5 558 patients from 15 RCTs who received bevacizumab had data for high-grade ATE for analysis (). The summary incidence of high-grade ATE was 2.0% (95% CI, 1.7–2.5).
Table IV. Incidence and relative risk (RR) of high-grade arterial thromboembolic events (ATE) with bevacizumab according to tumor types.
Grade 5 (fatal) ATE was rare. There were total seven deaths related to bevacizumab among these studies, with five due to cerebrovascular events from two studies [Citation22,Citation35], and two due to myocardial infarction from two studies [Citation21,Citation30].
Relative risk of ATE
The observed incidence of ATE associated with bevacizumab may be attributable to known or potential risk factors such as hypertension, diabetes, hyperlipidemia, chemotherapy, or malignancy. In order to determine the particular contribution of bevacizumab to the occurrence of ATE, and to exclude the influence of confounding factors, we calculated overall relative risk of ATE from these RCTs, in which a direct comparison was made between bevacizumab and a control with concurrent standard chemotherapy or biological therapy.
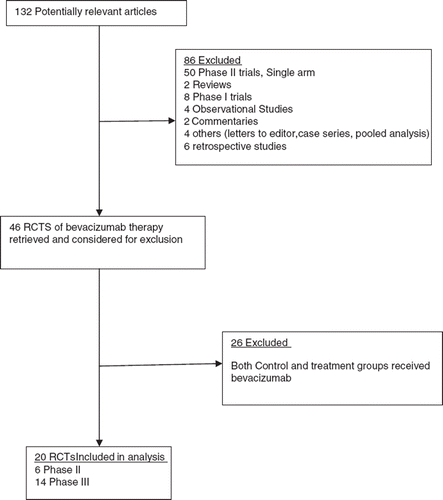
We combined the data of all-grade or high-grade ATE (if data for all grade not available) from each RCT, and performed a meta-analysis to calculate the overall RR associated with bevacizumab in comparison with controls. No heterogeneity was found among these studies included in the analysis despite clear disparity in tumor type and related treatment (). Using a fixed-effect model, the summary overall RR for bevacizumab versus control was 1.44 (95% CI 1.08–1.91, p=0.013). Thus, there was a significantly increased risk of developing ATE in patients treated with bevacizumab. The overall risk of ATE in patients receiving bevacizumab was 44% greater than control treatment.
Figure 2. Relative risk (RR) of arterial thromboembolic events (ATE) associated with bevacizumab versus controls. Overall summary RR of ATE was calculated using a fixed-effects model. RR for each study is displayed numerically on the left and graphically on the right. Total events and sample sizes are also displayed for each study. For study name, the first author’s name was used to represent each trial. If the same first author was involved in two trials, then the publication year was also included to identify the trial. For each trial the position of the square denoted incidence, horizontal lines represent 95% CI, and diamond plot represents overall results of the included trials. The size of the squares is directly proportional to the amount of data in each trial.
Also, we calculated summary RRs of all-grade and high-grade ATE with bevacizumab versus controls separately. As shown in , the summary RR of all grade ATE was 2.08 (95% CI 1.28–3.40, p=0.003) from eight RCTs, suggesting that bevacizumab significantly increased the risk of all-grade ATE by 108% when compared to a control. As shown in , the summary RR of high-grade ATE was 1.29 (95% CI 0.86–1.94, p = 0.21) from 15 RCTs, suggesting that bevacizumab might not increase the risk of high grade ATE significantly.
Influence of bevacizumab dose on the risk of ATE
We explored the relationship between the dose of bevacizumab and RR of ATE to further understand the role of bevacizumab in the development of ATE. A meta-analysis was performed to calculate the relative risk associated with bevacizumab at 2.5 or 5 mg/kg/week when compared to controls. The RR of ATE for bevacizumab at 2.5 mg/kg/week was 1.52 (95% CI 1.10–2.09) from nine RCTs including 7 116 patients (bevacizumab 3 581, controls 3 535). The RR of ATE at 5 mg/kg/week was 1.50 (95% CI 0.84–2.69) from 13 RCTs including 5 061 patients (bevacizumab 3 027, controls 2 934). Therefore, both high and low doses of bevacizumab increased the risk of ATE at a similar level.
Risk of ATE and tumor type
In order to investigate the relationship between ATE and tumor type, we determined the risk of ATE with bevacizumab according to tumor histology. The incidence and RR of all-grade ATE varied with different tumors (). The highest incidence of all-grade ATE was found in patients with CRC (6.1%, 95% CI, 4.4–8.5). Bevacizumab was also found to significantly increase the risk of all-grade ATE in patients with CRC (RR, 2.79, 95% CI 1.42–5.49, p=0.001) compared with controls.
The risk of high-grade ATE also varied with different tumors. Higher incidences of high-grade ATE were observed in patients with NSCLC, pancreatic cancer, and RCC (). Bevacizumab was found to significantly increase the risk of high-grade ATE in patients with RCC (RR, 5.14, 95% CI 1.35–19.64, p=0.029) in comparison with controls.
Risk of cardiac ischemia and stroke
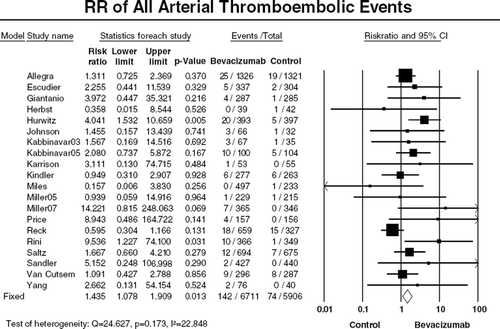
We also determined the risk of ATE separately according to anatomic sites of arteries (). Cardiac ischemia and stroke were the two main events for ATE. Five studies were available for analysis to calculate incidence and RR of cardiac events and stroke (). The summary incidence of high-grade cardiac ischemia was 1.5% (95% CI, 1.0–2.1%) among 2 322 patients receiving bevacizumab [Citation29]. The summary RR of developing high-grade cardiac ischemia with bevacizumab versus controls was 2.14 (95% CI, 1.12–4.08, p=0.021, ), suggesting that bevacizumab significantly increased the risk of high-grade cardiac ischemia by 114%.
Table V. Incidence and Relative Risk (RR) of arterial thromboembolic events (ATE) with bevacizumab according to organs.
Figure 3. Relative risk (RR) of cardiac ischemia or stroke associated with bevacizumab versus controls. Overall summary RRs of high grade cardiac ischemic events (A) or stroke (B) were calculated using a fixed-effects model. RR for each study is displayed numerically on the left and graphically on the right. Total events and sample sizes are also displayed for each study. For study name, the first author’s name was used to represent each trial. If the same first author was involved in two trials, then the publication year was also included to identify the trial. For each trial the position of the square denoted incidence, horizontal lines represent 95% CI, and diamond plot represents overall results of the included trials. The size of the squares is directly proportional to the amount of data in each trial.
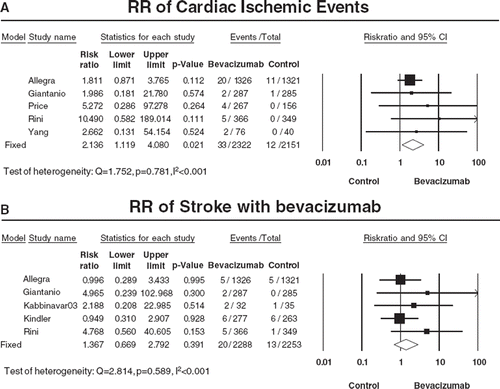
The summary incidence of ischemic stroke was 1.2% (95% CI: 0.8–1.9%) among 2 288 patients receiving bevacizumab from five RCTs, with the highest being 6.3% in one study [Citation27]. The summary RR of developing stroke with bevacizumab was 1.37 (95% CI 0.67–2.79, p=0.391) compared with controls (), suggesting no significant difference between bevacizumab and controls in the risk of stroke.
Discussion
This study showed that bevacizumab significantly increased the risk of developing ATE (RR: 1.58, 95% CI 1.09–2.28) in patients with a wide variety of advanced solid tumors from a meta-analysis of 20 RCTs, consistent with a previous pooled analysis of five RCTs [Citation12]. In addition, we showed that bevacizumab significantly increased the risk of cardiac ischemic events (RR: 2.14, 95% CI, 1.12–4.08, p=0.021). As bevacizumab is used extensively in routine cancer treatment and clinical trials, it is important for physicians to recognize the risk of ATE including cardiac ischemia associated with bevacizumab therapy, particularly in patients with pre-existing coronary cardiac disease. Further studies are recommended to determine risk factors and underlying mechanisms for risk reduction.
The role of bevacizumab in the development of ATE is not clear. The hallmark behind any ATE is the instability of atherosclerotic plaque and the associated activation of platelets. Bevacizumab may decrease an anti-inflammatory effect of chronic VEGF exposure, thus increasing the inflammation and atherosclerotic instability, leading to plaque rupture, platelet activation, and in situ thrombus formation [Citation38,Citation39]. Also, VEGF is important for the repair of endothelial cells and nitrous oxide (NO) production [Citation40]; the anti-VEGF effect of bevacizumab decreases endothelial cell renewal capacity, exposes pro-coagulant subendothelial tissue, decreases production of nitrous oxide, which is associated with vasoconstriction and increased platelet aggregation and adhesion to vascular endothelium [Citation41–43]. Additional mechanism may include a direct activation of platelets by bevacizumab through forming complexes with VEGF on platelets, as shown by Meyer et al. in transgenic mice [Citation44]. Finally, bevacizumab may interfere with the development of collateral circulation, a compensatory mechanism for obstructive arterial disease [Citation45].
Our study demonstrated that the risk of ATE associated with bevacizumab varies with tumor type, with a high risk seen in patients with RCC (RR, 5.14; 95% CI, 1.35–19.64, ). This variation may be related to multiple factors such as patient characteristics, tumor biology, and concurrent treatment. One possible explanation is that various ATE effects of bevacizumab may be affected by pretreatment VEGF levels in such patients; a higher level of VEGF may increase intra-plaque angiogenesis and thus predispose atherosclerotic plaque more dependent on VEGF for its stability. It was shown that the level of VEGF was highest in patients with RCC in comparison with other cancers [Citation46]. Another study demonstrated very high levels of VEGF among RCC patients.[Citation47]. In contrast, a very low level of VEGF was observed in patients with breast cancer [Citation48].
Our study showed that the risk of ATE did not vary with the dose of bevacizumab; RRs were 1.52 (95% CI, 1.10–2.09) and 1.50 (95% CI, 0.84–2.69) for bevacizumab at 2.5 and 5.0 mg/kg/week respectively. A lack of dose-effect relationship suggests that the low dose bevacizumab may be already reaching the saturation level to induce ATE; alternatively, the difference in effect may be too small to be detected. Interestingly, there was also no relationship between bevacizumab dose and the risk of venous thromboembolism, as shown by us previously [Citation17], suggesting that a similar mechanism involving anti-VEGF effect might exist underlying both arterial and venous thromboembolic events associated with bevacizumab.
This study showed that bevacizumab may increase the risk of ATE differentially according to the anatomic location of arteries. Based on the available data from five RCTs (), bevacizumab was associated with significantly increased risk of cardiac ischemia with an RR of 2.14 (95% CI, 1.12–4.08, p=0.021), but not stroke with an RR of 1.37 (95% CI, 0.67–2.79, p=0.39). This result suggests a possibility that bevacizumab may preferentially increase the risk of cardiac ischemic events. This could be the result of more prevalent atherosclerotic diseases in coronary arteries than in cerebral arteries; alternatively, coronary arteries may be more dependent on VEGF than cerebral arteries in maintaining vascular integrity, thus more prone to anti-VEGF treatment.
ATE may be a class-effect of angiogenesis inhibitors. Thalidomide, a potent anti- angiogenic agent blocking the action of VEGF and basic fibroblast growth factor, is associated with the occurrence of ATE, as reported in many case series [Citation49–51]. Sorafenib, an inhibitor of VEGF receptor (VEGFR), is associated with increased incidence of myocardial infarction compared to placebo in patients with hepatic cellular cancer or RCC [Citation52,Citation53]. Cardiovascular toxicity including acute coronary syndrome was also observed in patients treated with sunitinib, another inhibitor of VEGFR [Citation54]. Caution should be taken for an increased risk of ATE when these angiogenesis inhibitors are combined to enhance anti-tumor activity.
Prevention of ATE should be considered in high-risk patients receiving bevacizumab, including age old than 65 and a history of prior ATE [Citation12], or patients with CRC or RCC according to our study. The use of ASA, which did not significantly increase the risk of bleeding [Citation12], should be considered in these patients if they do not have contraindications.
Our study has several limitations. Firstly, three versions of NCI-CTC were used to grade adverse events, and may have inconsistency in reported events of ATE. However, 18 of 20 studies used versions 2 and 3, which are very similar. In addition, the significance of low-grade events (grade 1-2) is not clear, and baseline cardiovascular condition was not specified. Secondly, all the studies were conducted at various institutions, and the ability to detect an ATE can vary among the institutions, which could result in potential bias of recording adverse events. However, the reported incidences of ATE are relatively similar across these studies; in addition, the calculation of RRs may reduce inherent bias within each study, and RRs reported by all of the studies were remarkably non-heterogenous. Thirdly, these studies were conducted at academic centers and major research institutions, and patients in these clinical trials had adequate major organ function, which may not reflect general patient population in a community or patients with organ dysfunctions. Fourthly, there could be a potential observation time bias because bevacizumab is often associated with prolonged progression-free survival. However, most ATE events occurred in the early phase of treatment; the median time to the first event was 2.6 months in the bevacizumab-treated group versus 2.1 months in the control group [Citation12]. Finally, this is a meta-analysis at the study level, so confounding factors and specific risk factors at the patient level cannot be assessed, and incorporated into the analysis.
In conclusion, the addition of the commonly used angiogenesis inhibitor bevacizumab to standard anti-neoplastic therapy significantly increased the risk of ATE particularly cardiac ischemia in cancer patients. The risk is similarly increased in patients receiving both low and high doses of bevacizumab, and may vary with tumor type, with higher risks being associated with RCC or CRC. It is important for physicians and patients to recognize the cardiovascular side effect with bevacizumab. Future studies are recommended to investigate risk reduction.
Acknowledgements
Shenhong. Wu. is partially supported by the Research Foundation of SUNY. Shenhong. Wu. received honoraria from Onyx Pharmaceuticals, Novartis, and Wyeth, and a speaker for Onyx, Pfizer, and Novartis. Shenhong. Wu. had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cavallaro U, Christofori G. Molecular mechanisms of tumor angiogenesis and tumor progression. J Neurooncol 2000; 50:63–70.
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29(6 Suppl 16):15–8.
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27.
- Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 2005; 65:671–80.
- Genentech BioOncology. Avastin (bevacizumab), product insert. Last accessed on June, 2009 [cited; Available from: http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf
- Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Colangelo LH, Lopa SH, . Initial safety report of NSABP C-08: A randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol 2009; 27:3385–90.
- Karrison T, Kindler HL, Gandara DR, Lu C, Guterz TL, Nichols K, . Final analysis of a multi-center, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioma (MM). ASCO Meeting Abstracts 2007;25(18 Suppl):7526.
- Kindler HL, Niedzwiecki D, Hollis D, Oraefo E, Schrag D, Hurwitz H, . A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB. ASCO Meeting Abstracts 2007;25(18 Suppl):4508.
- Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, . Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol 2008;26:1830–5.
- Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology 2005; 69(Suppl 3):25–33.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, . Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007; 99:1232–9.
- Meade MO, Richardson WS. Selecting and appraising studies for a systematic review. Ann Intern Med 1997;127:531–7.
- National Cancer institute’s common toxicity criteria (version 2). Last accessed on March, 2009. 1999 [cited; Available from: http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf
- National Cancer institute’s common toxicity criteria (version 3). Last accessed on March, 2009. 2006 [cited; Available from: http://ctep.cancer.gov/forms/CTCAEv3.pdf
- Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: A meta-analysis. Lancet Oncol 2009;10:559–68.
- Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA 2008;300:2277–85.
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 2007;49:186–93.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.
- Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med 1997;127:820–6.
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, . Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet 2007;370(9605): 2103–11.
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, . Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231–7.
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, . Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009;27:1227–34.
- Miles D, Chan A, Romieu G, Dirix LY, Cortes J, Pivot X, . Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. ASCO Meeting Abstracts 2008;26(15 Suppl):LBA1011.
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, . Bevacizumab in combination with fluorouracil and leucovorin: An active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005;23:3502–8.
- Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, . Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–9.
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, . Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003;21:60–5.
- Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, . Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 2004;22:2184–91.
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, . A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–34.
- Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, . Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J Clin Oncol 2005; 23:3697–705.
- Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, . Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539–44.
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, . Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26: 5422–8.
- Price TJ, Gebski V, van Hazel GA, Robinson BA, Broad A, Ganju V, . International multi-centre randomised Phase II/III study of Capecitabine (Cap), bevacizumab (Bev) and mitomycin C (MMC) as first-line treatment for metastatic colorectal cancer (mCRC): Final safety analysis of the AGITG MAX trial. ASCO Meeting Abstracts 2008;26 (15 Suppl):4029.
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, . Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357: 2666–76.
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, . Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355: 2542–50.
- Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, . Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743–50.
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, . Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005;23:792–9.
- Kuenen BC, Levi M, Meijers JC, Kakkar AK, van Hinsbergh VW, Kostense PJ, . Analysis of coagulation cascade and endothelial cell activation during inhibition of vascular endothelial growth factor/vascular endothelial growth factor receptor pathway in cancer patients. Arterioscler Thromb Vasc Biol 2002;22:1500–5.
- Hesser BA, Liang XH, Camenisch G, Yang S, Lewin DA, Scheller R, . Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood 2004;104:149–58.
- Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol 2001;280:C1375–86.
- Kilickap S, Abali H, Celik I. Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol 2003;21:3542; author reply 3.
- Yao SK, Ober JC, Krishnaswami A, Ferguson JJ, Anderson HV, Golino P, . Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation 1992;86: 1302–9.
- de Graaf JC, Banga JD, Moncada S, Palmer RM, de Groot PG, Sixma JJ. Nitric oxide functions as an inhibitor of platelet adhesion under flow conditions. Circulation 1992;85: 2284–90.
- Meyer T, Robles-Carrillo L, Robson T, Langer F, Desai H, Davila M, . Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost 2009;7:171–81.
- Pereg D, Lishner M. Bevacizumab treatment for cancer patients with cardiovascular disease: A double edged sword? Eur Heart J 2008;29:2325–6.
- Bando H, Brokelmann M, Toi M, Alitalo K, Sleeman JP, Sipos B, . Immunodetection and quantification of vascular endothelial growth factor receptor-3 in human malignant tumor tissues. Int J Cancer 2004;111:184–91.
- Schips L, Dalpiaz O, Lipsky K, Langner C, Rehak P, Puerstner P, . Serum levels of vascular endothelial growth factor (VEGF) and endostatin in renal cell carcinoma patients compared to a control group. Eur Urol 2007;51: 168–73; Discussion 74.
- Weich HA, Bando H, Brokelmann M, Baumann P, Toi M, Barleon B, . Quantification of vascular endothelial growth factor-C (VEGF-C) by a novel ELISA. J Immunol Methods 2004;285:145–55.
- Alkindi S, Dennison D, Pathare A. Arterial and venous thrombotic complications with thalidomide in multiple myeloma. Arch Med Res 2008;39:257–8.
- Scarpace SL, Hahn T, Roy H, Brown K, Paplham P, Chanan-Khan A, . Arterial thrombosis in four patients treated with thalidomide. Leuk Lymphoma 2005;46:239–42.
- Altintas A, Ayyildiz O, Atay AE, Cil T, Isikdogan A, Muftuoglu E. Thalidomide-associated arterial thrombosis: Two case reports. Ann Acad Med Singapore 2007; 36: 304–6.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90.
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, . Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34.
- Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, . Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008;26:5204–12.