Abstract
Background. Previous studies have shown that there have been systematic differences between the Nordic countries in population-based relative survival of patients with respiratory cancer (lung, pleura, larynx, nose and sinuses). Material and methods. Relative survival of patients with respiratory cancer diagnosed in the Nordic countries in 1964–2003 and followed up to the end of 2006 was studied and contrasted with developments in incidence and mortality. Results. For cancer of the lung, relative survival is lower in Danish patients than in the other countries during the first months of follow-up after diagnosis. For cancer of pleura, the relative survival ratios indicate that there may be problems in the official coding of the causes of death in Denmark, Norway and Sweden. There has been little improvement in survival of patients with cancer of the respiratory organs in the Nordic countries over time. Conclusions. The slightly lower survival of Danish lung cancer patients may be related to a less favourable stage distribution and to an increased prevalence of causal factors, affecting the mortality due to competing risks of death. A reclassification of official causes of death at the cancer registry may be needed for cancer of the pleura in order to make the corresponding mortality rates comparable between countries.
Lung cancer is the second most common cancer in both men and women in the Nordic countries combined, with rates only superseded by prostate and breast cancer, respectively, while it is the most common cause of cancer death. The annual number of incident cancers in 1999–2003 was more than 11 000 and there were more than 10 000 deaths [Citation1]. Of all cancer cases diagnosed in the Nordic countries (excluding non-melanoma skin) in 1999–2003, 11.1% in males and 7.4% in females were located at the lung. The main risk factor is tobacco smoking [Citation2]. The age-standardised 5-year relative survival ratios have diverged 1958–1987 with the disparity in survival increasing in Denmark relative to the other countries [Citation3]. The most important underlying factor for these differences is likely the unfavourable stage distribution in Denmark, but differences in comorbidity due to the high prevalence of smoking in Denmark [Citation2] could also play a role [Citation4]. The recent EUROCARE-4 study showed that the 5-year relative survival ratios in Iceland and Sweden were amongst the highest in Europe, whereas the Danish patient survival was amongst the lowest [Citation5–7].
Cancer of the pleura is one of the more rare cancer sites with 360 cases diagnosed per year in the Nordic countries in the period 1999–2003, constituting 0.5% of the male cancer cases and 0.1% of the female cancers. The numbers of deaths reported over the same period were 220 per year [Citation1]. The main risk factor is asbestos with multiplicative effects in combination with tobacco smoking [Citation8]. The cancer occurs commonly in older persons aged over 75, and is seldomly observed before the age of 50. There have been few sets of comparative Nordic relative survival data published. The EUROCARE-4 study reported that for patients diagnosed in 1995–1999, the age-standardised 5-year relative survival ratios were 7.5% and 3.5% for Finland and Norway, respectively [Citation7]. In Denmark and Sweden, only 1-year relative survival could be calculated due to few numbers across age strata. Overall, the European data reported low relative survival ratios that did not significantly differ by sex (6.5% in males, 10.1% in females).
Larynx cancer is a relatively rare cancer in the Nordic countries but one that is markedly more common among men than women. During the period 1999–2003, 1% of all new cancers were located at the larynx, with around 85% diagnosed among males. Rates in this period differed between the Nordic countries, with the highest incidence rates seen in men in Denmark (4.6 per 100 000) and women in Iceland (1.3) while the lowest rates were observed in Swedish men (2.0) and Finnish women (0.2) [Citation1]. The main risk factors are tobacco smoking, alcohol drinking, and the interaction between these two habits [Citation9]. The 5-year relative survival in patients diagnosed 1995–1999 varied considerably between the Nordic countries, from 58% in Iceland to 71% in Sweden [Citation7]. In a Nordic study of relative survival trends 1958–1987, the 5-year age-standardised survival among male larynx cancer patients was close to 60% throughout the study period 1958–1987 except for a low Finnish estimate before 1968 [Citation3].
Cancers of the nose, nasal cavities, middle ear and accessory sinuses are collectively a rare cancer form in most western countries, with world age-standardised rates in the Nordic countries less than 1 per 100 000. In 1999–2003, these cancers constituted about 0.2% in males and females in the Nordic countries. The incidence is lower among women than men [Citation1], possibly a result of a differential in the prevalence of certain occupational and lifestyle exposures including wood dust and cigarette smoke; ionising radiation (thorotrast and radon) may also elevate the risk [Citation10]. Five-year relative survival compilations of European survival for patients diagnosed during the years 1995–1999 have reported large variations in the Nordic countries, with ratios ranging from 41% in Finland through to 61% in Sweden [Citation7].
In this study, the relative survival and excess mortality observed in the Nordic patients in 1964–2003 is contrasted with developments seen in the incidence and mortality rates over the same period. The aim is to provide some insight into the developments observed.
Material and methods
Lung cancer was defined as the ICD-10 code C33-34, cancer of the pleura as C38, cancer of the larynx as C32 and that of the nose and sinuses as C30-31. The materials and methods are described in detail in an earlier article in this issue [Citation11]. In brief, the NORDCAN database contains comparable data on cancer incidence and mortality in the Nordic countries, as delivered by the national cancer registries, with follow-up information on death or emigration for each cancer patient available up to and including 2006.
Sex-specific 5-year relative survival was calculated for each of the diagnostic groups in each country for eight 5-year periods from 1964–1968 to 1999–2003. For the last 5-year period, so-called hybrid methods were used. Country-specific population mortality rates were used for calculating the expected survival. Age-standardisation used the weights of the International Cancer Survival Standard (ICSS) cancer patient populations [Citation12]. We present age-standardised (World) incidence and mortality rates, 5-year relative survival, and excess mortality rates for the follow-up periods of within one month, 1–3 months and 2–5 years following diagnosis, as well as age-specific 5-year relative survival by country, sex and 5-year period.
Results
Cancer of the lung
Incidence and mortality. Denmark had the highest age-standardised incidence (45 per 100 000) in males in 2003, after a large reduction in incidence in Finland from 79 in 1970 to 36 per 100 000 three decades later (). The most recent incidence rates place Sweden as the lowest risk country. The incidence rates in males have been decreasing in Finland since 1970, in Denmark since 1985, and in Sweden since 1980, whereas the rates have, after an initial increase remained stable in Iceland and Norway since the mid-1980s. The incidence rates in females have increased in each of the Nordic countries. The increase is strongest in Denmark and Iceland and weakest in Finland. The mortality trends closely followed those of incidence reflecting the very unfavourable survival of the patients in general ().
Figure 1. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for patients of lung cancer by sex and country. Nordic cancer survival study 1964–2003.
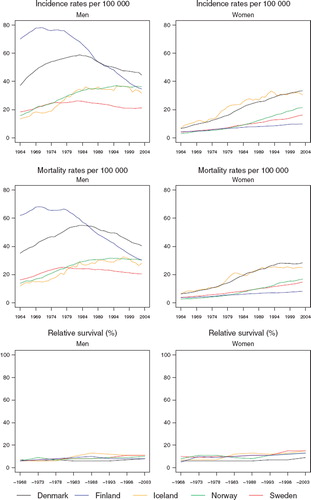
Survival. There were few differences in the age-standardised 5-year relative survival ratios between countries (, ). The Danish ratios were inferior to those in the other Nordic countries, particularly in females. The ratios were slightly higher in females than in males and in general they increased slowly over time. In the last 15 years, however, no change in survival could be observed for males in Finland or Iceland.
Table I. Trends in survival for lung cancer by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
The improvement in relative survival was partly attributable to a decrease in the excess death rates of the patients during the first three months after diagnosis (). The inter-country differences were also partially explained by survival differences in these early months of follow-up. The 5-year relative survival was markedly higher in younger patients irrespective of population (). In general, few notable changes were observable over time.
Figure 2. Trends in age-standardised (ICSS) excess death rates per 100 person years for lung cancer by sex, country, and time since diagnosis. Nordic cancer survival study 1964–2003.
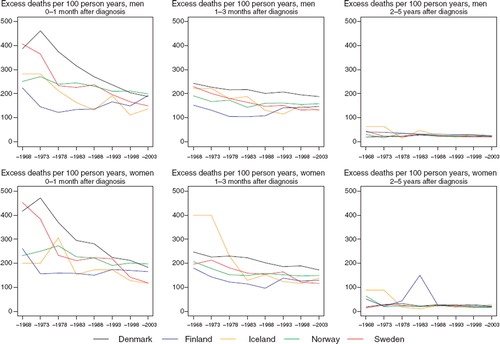
Table II. Trends in 5-year age-specific relative survival in percent after lung cancer by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the pleura
Incidence and mortality. There has been a strong increase in the incidence in males in all countries, whereas female rates have been fairly constant over the last two decades (). Of the Nordic countries, cancer of the pleura has tended to be most common in Denmark in both sexes. In males, Norway has been catching up with Denmark during the most recent two decades. In Swedish males – and possibly also in Icelandic males – the incidence has not increased in the most recent decade. In females, the higher Danish rates have been decreasing since the mid-1970s and currently they equal the rates observed among Finnish females. The age-standardised incidence rates for 1999–2003 ranged in males from 0.8 per 100 000 (Iceland) to 1.7 (Denmark), and in females between 0.1 and 0.3 per 100 000.
Figure 3. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for patients of cancer of the pleura by sex and country. Nordic cancer survival study 1964–2003.
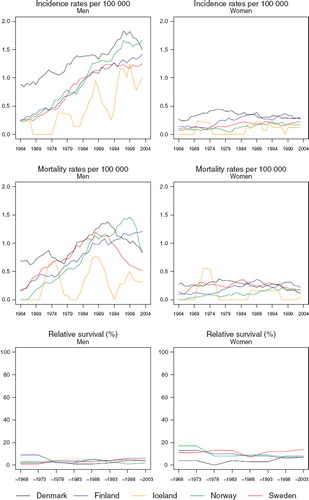
The mortality rates in recent years convey a very different temporal pattern compared to that of incidence in Denmark, Norway and Sweden. There have been substantial decreases in Denmark and Sweden since 1990 and in Norway since 1999. In females, however, the changes are less apparent with time. The decreases in mortality in women in Denmark and Sweden are some five years ahead of the corresponding rates in men, but both exhibit a similar rate of decline.
Survival. The declines in mortality in males do not seem to be followed or accompanied by increments in 5-year relative survival in Denmark or Sweden (, ). The age-standardised 5-year relative survival ratios have in any case remained low, between 2% and 6% in males and between 7% and 14% in females. The ratios in Swedish female patients have had a tendency to be higher than those in female patients in the other Nordic countries. The Icelandic numbers are too small to allow estimation of relative survival ratios.
Table III. Trends in survival for cancer of the pleura by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
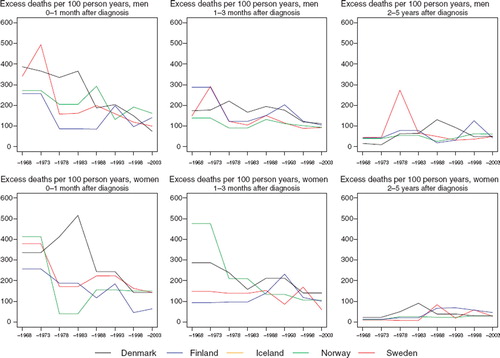
The excess death rates of the patients during the first three months have tended to decline with calendar time (). No systematic differences between countries can be seen, in part related to the inherent random variation associated with these estimations.
Figure 4. Trends in age-standardised (ICSS) excess death rates per 100 person years for cancer of the pleura by sex, country, and time since diagnosis. Nordic cancer survival study 1964–2003. No Icelandic curves. Too few partients to calculate survival for Iceland.
The age-specific trends in 5-year survival are hard to interpret because of the few numbers involved (). In Swedish and Finnish females, the younger patients appear to have a higher relative survival than older patients.
Table IV. Trends in 5-year age-specific relative survival in percent after cancer of the pleura by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the larynx
Incidence and mortality. Among men, the incidence rates varied between countries and over time. Finland started with the highest incidence of about 7 per 100 000, but the rates steadily decreased over the period to around 2 by 2003 (). In all the other countries, rates increased initially but then reached a plateau and subsequently fell, although the calendar year at which the decline began to emerge varied considerably by population. To illustrate, rates began to fall in the mid-1970s in Sweden but as late as the mid-1990s in Denmark.
Figure 5. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for patients of laryngeal cancer by sex and country. Nordic cancer survival study 1964–2003.
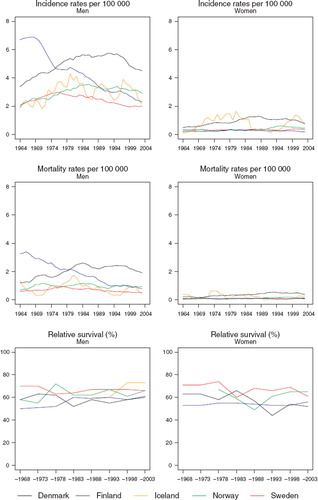
Among women, rates appear rather flat on this scale, although evidently rates are higher and were increasing in women in Denmark in a manner similar to that observed in men. The mortality patterns reflect those of incidence, although the absolute level of rates is diminished by about one-half ().
Survival. Any increases in 5-year relative survival among men are very slight over the study years, while the survival patterns among women are quite flat (). Sweden appears most consistently as the country with the highest survival in both sexes and over time ().
Table V. Trends in survival for laryngeal cancer by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Among men, the most substantive variation in excess mortality is within the first three months, but compared to many other sites the excess rates are not very high (). The comparison is less meaningful in women given the rarity of the disease and the random variation inherent. The age-specific time trends are based on small numbers of patients (). The relative survival tends to be lower in older patients.
Figure 6. Trends in age-standardised (ICSS) excess death rates per 100 person years for laryngeal cancer by sex, country, and time since diagnosis. Nordic cancer survival study 1964–2003.
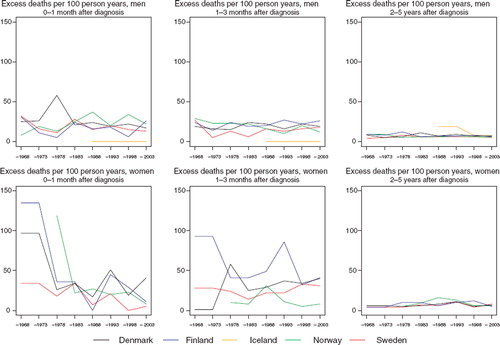
Table VI. Trends in 5-year age-specific relative survival in percent after laryngeal cancer by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the nose and sinuses
Incidence and mortality. As an uncommon set of tumours, there is considerable random variation in the trends in the underlying rates (). The incidence rates tend to be rather stable or slightly increasing with time in Denmark, at least in men.
Figure 7. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for patients of cancer of the nose and sinuses by sex and country. Nordic cancer survival study 1964–2003.
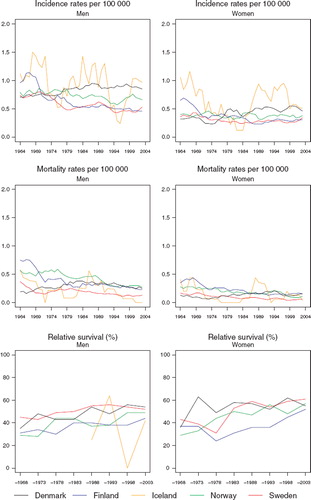
In 2003, the rate in Danish males, 0.9 per 100 000 was almost double of that in Finland. Mortality trends exhibit mainly consistent, minor declines in all countries. Of note is an earlier increase in Denmark, observed for men and women until the early-1980s.
Survival. In general, increases in 5-year relative survival ratios are observed over time in the Nordic countries, although there is some heterogeneity in the extent of the increase, ranging from 10 to 20 percentage points (). Variations in excess mortality are most apparent among males in the first year after diagnosis between Denmark and the other countries (). The age-specific trends are based on small numbers (). The relative survival ratios tend to be higher in younger patients, particularly in Finland, Norway and Sweden.
Figure 8. Trends in age-standardised (ICSS) excess death rates per 100 person years for cancer of the nose and sinuses by sex, country, and time since diagnosis. Nordic cancer survival study 1964–2003.
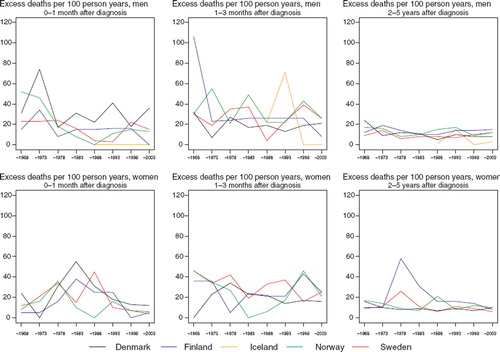
Table VII. Trends in survival for cancer of the nose and sinuses by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table VIII. Trends in 5-year age-specific relative survival in percent after cancer of the nose and sinuses by sex and country. Nordic cancer survival study 1964–2003.
Discussion
Respiratory cancer survival is relatively poor and particularly low for cancers of the lung and pleura. Male patients, elderly patients, and patients diagnosed in Denmark are the broad groups that tended to have lower survival than others.
The lower relative survival ratios for lung cancer in Denmark have been related to an unfavourable stage distribution, whereas differences between morphologic types have not been able to explain the survival differences between countries [Citation4]. Patients diagnosed with small cell carcinoma have an unfavourable survival compared to the other patients. As the stage and histological type-specific differences in relative survival between the Nordic countries are much smaller, it is likely that the delays in diagnosis can play an important role. These delays can be related to both the patient and the health services.
Accounting for differences in general (competing-risk) mortality by educational level in Denmark made the relative survival ratios by educational level approximately equal [Citation13]. As smoking is more common in Denmark than in the other Nordic countries, smoking-related co-morbidity may worsen the Danish patients’ survival compared to that of the other Nordic patients. Stopping smoking is thus not only important in lung cancer prevention, but it is also important in patient survival as a factor leading to a decreased co-morbidity of these patients.
The early excess mortality has been decreasing, and the differences between the Nordic countries in this respect have diminished as well. More rapid diagnostics and less postoperative deaths may play an important role in this [Citation14]. Clearly, these improvements are not as yet impacting on 5-year relative survival.
For cancer of the pleura, Danish, Swedish and possibly also Norwegian men appear to have favourable mortality developments without evidence of increasing survival. This could possibly relate to a subset of deaths due to cancer of the pleura being misclassified, for example, as cancers of the lung. The mortality rates are sensitive to these kinds of changes, although relative survival will be unaffected since it is not based on the coding of individual causes of death. In Finland, the official causes of death are reclassified by the Cancer Registry based on multiple information sources (on average, there are five notifications per case) available for each patient so such a phenomenon is not likely to occur [Citation15].
The high survival of female patients in Sweden may in part relate to a lack of death certificate-initiated cases in Sweden. These cases normally have an inferior survival compared to the other registered cases [Citation16].
Incidence and mortality trends of laryngeal cancer are likely to reflect the cohort-driven independent and interactive risks associated with high levels of tobacco and alcohol consumption in each of the Nordic populations. Finnish men started to smoke earlier than Danish men, but effective smoking cessation campaigns started earlier in Finland [Citation17] with marked decreasing trends in lung cancer incidence observed in males. The proportion of smokers in Sweden has been lower than in the other countries [Citation18]. Alcohol consumption increased in Denmark up to mid-1980s with less strict regulations on the purchase of beer, wine and alcohol than in force in the other Nordic countries [Citation19].
That the trends in incidence and mortality are rather similar between countries implies, among other explanations, a general lack of improvement in prognosis, and indeed the survival estimates do not show any marked increases over time, an observation in keeping with those reported by EUROCARE-4 across Europe [Citation20]. While Denmark and Finland had the lowest survival in both sexes, it is unclear whether there are genuine survival differences in the Nordic countries, and where they exist, the extent to which competing causes of death operate as a function of the prevalence and distribution of these risk factors. While larynx-preserving techniques are considered one of the most important achievements in head and neck oncology [Citation21], their collective impact on survival as opposed to life quality at the population level over the last decades appears rather minor in the Nordic countries. Given these survival trends and the fact that the vast majority of laryngeal cancer patients are current or ex-smokers, however, the role of primary prevention cannot be understated.
Cancers of the nose, nasal cavities, middle ear and accessory sinuses are rare neoplasms, with occupational exposures and smoking considered as the dominant aetiological factors [Citation10] with prognosis dependent on localisation, stage, and treatment modality [Citation22,Citation23]. The stable incidence, steady declines in mortality and increasing survival across the Nordic countries imply modest improvements in the quality of care, which may include improving treatment modalities [Citation24].
Relatively little improvement has thus far been achieved in improving the prognosis of respiratory cancer patients, in spite of many clinical actions and possibilities [Citation14,Citation24,Citation25]. Early detection through screening interventions has been shown to be ineffective at the population level [Citation26]. Primary prevention strategies therefore that promote smoking avoidance and cessation remain key actions not only in terms of cancer control as a whole, but specifically in enabling improvements in cancer patient survival.
Acknowledgement
The Nordic Cancer Union (NCU) has financially supported the development of the NORDCAN database and program, and the analyses in the survival project.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Engholm G, Ferlay J, Christensen N, Bray F, Klint Å, Ólafsdóttir E, . NORDCAN: Cancer incidence, mortality and prevalence in the Nordic countries, Version 3.2. Association of Nordic Cancer Registries. Danish Cancer Society (http://www.ancr.nu); 2008.
- Dreyer L, Winther JF, Pukkala E, Andersen A. Tobacco smoking. Olsen JH, Andersen A, Dreyer L, Pukkala E, Tryggvadottir L, Gerhadsson de Verdier M, . Avoidable cancers in the Nordic countries. APMIS 1997;105 (Suppl 76):9–47.
- Engeland A, Haldorsen T, Tretli S, Hakulinen T, Hörte LG, Luostarinen T, . Prediction of cancer mortality in the Nordic countries up to the years 2000 and 2010, on the basis of relative survival analysis. A collaborative study of the five Nordic cancer registries. APMIS 1995;103(Suppl 49): 1–163.
- Storm HH, Dickman PW, Engeland A, Haldorsen T, Hakulinen T. Do morphology and stage explain the inferior lung cancer survival in Denmark? Eur Respir J 1999; 13: 430–5.
- Berrino F, De Angelis R, Sant M, Rosso S, Lasota MB, Coebergh JW, . Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: Results of the EUROCARE-4 study. Lancet Oncol 2007; 8:773–83.
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, . Recent cancer survival in Europe: A 2000–02 period analysis of EUROCARE-4 data. Lancet Oncol 2007;8:784–96.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, . EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Becklake MR, Bagatin E, Neder JA. Asbestos-related diseases of the lungs and pleura: Uses, trends and management over the last century. Int J Tuberc Lung Dis 2007;11:356–69, 2008;12:824.
- Bofetta P, Trichopolous D. Cancer of the lung, larynx and pleura. Adami H-O, Hunter D, Trichopolous D. Textbook of cancer epidemiology. 2nd. Oxford: Oxford University Press; 2008. 349–77.
- Littman AJ, Vaughan TL. Cancers of the nasal cavity and paranasal sinuses. Schottenfeld D, Fraumeni JF Jr. Cancer epidemiology and prevention. 3rd. Oxford: Oxford University Press; 2006. 603–19.
- Engholm G, Gislum M, Bray F, Hakulinen T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 and followed up to the end of 2006. Material and methods. Acta Oncol 2010 (submitted).
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Dalton SO, Steding-Jessen M, Engholm G, Schüz J, Olsen JH. Social inequality and incidence of and survival from lung cancer in a population-based study in Denmark, 1994–2003. Eur J Cancer 2008;44:1989–95.
- Zee YK, Eisen T. Survival from lung cancer in England and Wales up to 2001. Br J Cancer 2008;99:S43–S46.
- Hakulinen T, Teppo L. Causes of death among female patients with cancer of the breast and intestines. Ann Clin Res 1977;9:15–24.
- Robinson D, Sankila R, Hakulinen T, Møller H. Interpreting international comparisons of cancer survival: The effects of incomplete registration and the presence of death certificate only cases on survival estimates. Eur J Cancer 2007;43: 909–13.
- Hakulinen T, Pukkala E, Kenward M, Teppo L, Puska P, Tuomilehto J, . Changes in cancer incidence in North Karelia, an area with a comprehensive preventive cardiovascular programme. IARC Sci Publ 1990;103:133–48.
- Forey B, Hamling J, Lee P, Wald N. International smoking statistics. Oxford: Oxford University Press; 2002.
- Thorsen T. One hundred years of alcohol abuse: Alcohol consumption and alcohol problems in Denmark. Copenhagen: Danish Council on Alcohol and Narcotics; 1990 (in Danish).
- Verdecchia A, Guzzinati S, Francisci S, De Angelis R, Bray F, Allemani C, . Survival trends in European cancer patients diagnosed in 1988 to 1999. Eur J Cancer 2009; 45:1042–66.
- Lefebvre JL, Rolland F, Tesselaar M, Bardet E, Leemans CR, Geoffrois L, . Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst 2009;101:142–52.
- Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: Are we making progress? A series of 220 patients and a systematic review. Cancer 2001;92:3012–29.
- Grau C, Jakobsen MH, Harbo G, Svane-Knudsen V, Wedervang K, Larsen SK, . Sino-nasal cancer in Denmark 1982–1991 – a nationwide survey. Acta Oncol 2001;40:19–23.
- Nutting CM, Robinson M, Birchall M. Survival from laryngeal cancer in England and Wales up to 2001. Br J Cancer 2008;99:S38–S39.
- Coebergh JW, Albrecht T. Cancer control in Europe. State of the art in 2008. Eur J Cancer 2008;44: 1341–475.
- Hakama M, Coleman MP, Alexe D-M, Auvinen A. Cancer screening: Evidence and practice in Europe 2008. Eur J Cancer 2008;44:1404–13.