Abstract
Background. This is the first comprehensive population-based study on relative survival of lip, oral cavity and pharyngeal cancer in the Nordic countries. Material and methods. Relative survival of patients with cancers of the lip, oral cavity, and pharynx diagnosed in the Nordic countries in 1964–2003 and followed up to the end of 2006 was studied and contrasted with trends in incidence and mortality. Results. There are marked differences in incidence between countries and over time. The stability of the relative survival ratios gives support to the hypothesis that the incidence differences are more likely to be real and not materially affected by differences in definitions and coding. Of particular note are the steep rises in pharyngeal cancer incidence in Denmark in both sexes. Survival has only moderately improved over time and has tended to be slightly higher in females than males. Conclusions. Co-morbidity caused by smoking and high alcohol consumption are likely to be partially responsible for differences between countries. Advances in therapy and standards of care are also likely to have played a role in the increasing survival trends.
In the Nordic countries, cancers of the lip, oral cavity, and pharynx are relatively rare, accounting for about 2% of all new cancer cases observed in the Nordic countries in 2002–2006 [Citation1]. The main known risk factors are smoking, oral tobacco consumption and alcohol, but the role of these factors varies greatly between the anatomic locations and sexes [Citation2]. These risk factors, particularly smoking, result in patient co-morbidity which may impact on the relative survival differences between population groups. The role of HPV infection has been established as a risk factor, particularly for oropharyngeal cancer [Citation3].
Rather little has been published thus far on the population-based survival experience of head and neck cancer patients in the Nordic countries. The EUROCARE studies [Citation4,Citation5] have shown that the Nordic relative survival figures (with the occasional exception of Denmark) are among the highest observed in Europe.
The aim of the present study is to describe and compare Nordic trends in relative survival and excess mortality of the patients from 1964–2003 to those of incidence and mortality, providing some basic interpretation of the findings.
Material and methods
The materials and methods are described in detail in an earlier article in this issue [Citation6]. In brief, the NORDCAN database contains comparable data on cancer incidence and mortality in the Nordic countries, as delivered by the national cancer registries, with follow-up information on death or emigration for each cancer patient available up to and including 2006. Oral cavity and pharyngeal tumours were extracted according to ICD-10 for cancers of the lip (ICD-10 C00), tongue (ICD-10 C01-02), salivary glands (ICD-10 C07-08), mouth (ICD-10 C03-06), and pharynx (ICD-10 C09-14).
Sex-specific 5-year relative survival was calculated for each of the diagnostic groups in each country for eight 5-year periods from 1964–1968 to 1999–2003. For the last 5-year period, so-called hybrid methods were used. Country-specific population mortality rates were used for calculating the expected survival. Age-standardisation used the weights of the International Cancer Survival Standard (ICSS) cancer patient populations [Citation7]. We present age-standardised (World) incidence and mortality rates, 5-year relative survival, and excess mortality rates for the follow-up periods of within one month, 1–3 months and 2–5 years following diagnosis, as well as age-specific 5-year relative survival by country, sex and 5-year period. Except for lip cancer, age-specific 5-year relative survival is not shown for the Icelandic patients because of the small number of patients involved.
Results
Cancer of the lip
Incidence and mortality. The age-standardised incidence of lip cancer in males has been in a marked and steady decline in all the Nordic countries for decades (). In 1999–2003, the observed decrease in Iceland appeared to cease, but this may also be due to the inherent random variation. In 2003, the incidence rates were all between 1.0 and 1.6 per 100 000, the lowest in Sweden and the highest in Finland. At the beginning of the observation period, the incidence rates in females were considerably lower than those in males but, with declining rates in males and slightly increasing rates in females, the rates are approaching each other. Mortality from lip cancer is quite minimal (); the average annual number of deaths in the Nordic countries in 1999–2003 due to this cancer being 17 and six in males and females, respectively. The mortality rates in males have decreased in the Nordic countries and appear to be approaching zero. In females, mortality rates have been consistently close to zero, on account of the rarity of the disease and the good prognosis of patients.
Figure 1. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for lip cancer by sex and country. Nordic cancer survival study 1964–2003.
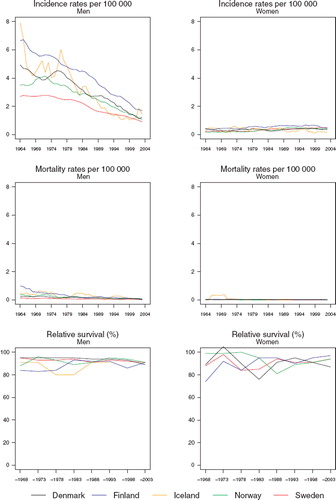
Survival. The age-standardised 5-year relative survival ratios have been between 90% and 100% (, ). Due to limited numbers the Icelandic female ratio could not be evaluated, and the figures presented for males are affected by random variation.
Table I. Trends in survival for lip cancer by sex and country. Number of tumours (N) included and the 5-year age- standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
In males, the excess death rates were higher in the first follow-up month than in the subsequent periods of follow-up (). There were no systematic inter-country differences. There was a tendency to observe somewhat poorer relative survival in patients aged 70 years or more (). Some of the rates are higher than 100% due to zero observed deaths in the patient groups. This can result by chance where excess mortality is small or non-existent and the patient groups are small.
Figure 2. Trends in age-standardised (ICSS) excess death rates per 100 person years for lip cancer by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003.
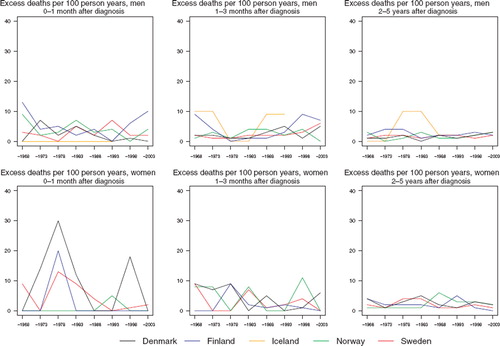
Table II. Trends in 5-year age-specific relative survival in percent after lip cancer by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the tongue
Incidence and mortality. The incidence has increased quite rapidly in males during the entire period (). During the last decade, the Danish rates have accelerated to a level much higher than those in the other countries. The Icelandic rates have been consistently the lowest among men in the Nordic countries, where age-standardised incidence rates range from 0.8 (Iceland) to 1.9 per 100 000 (Denmark) during the period 1999–2003. In females, there is very little variation between the countries during the most recent two decades, although the Finnish rates have had a tendency of being higher than those in the other countries. In Iceland, the rates before 1980 were systematically higher in females than in males but the underlying numbers are small. The mortality rates show a much more modest increase in Danish males than seen in the incidence rates, while in the Norwegian trends, the mortality decrease apparent in men during 1994–2003, was not observed for incidence ().
Figure 3. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for cancer of the tongue by sex and country. Nordic cancer survival study 1964–2003.
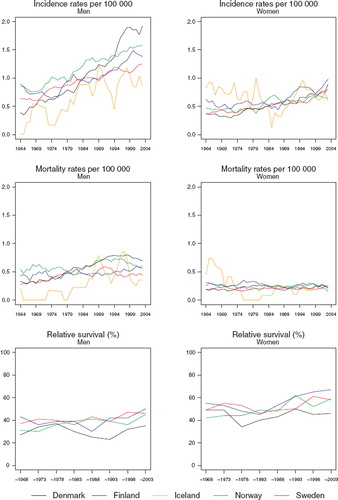
Survival. The age-standardised 5-year relative survival ratios in Denmark have during the three last decades been below those observed in the other Nordic countries (, ). Female patients tend to have higher survival compared to men, while the increases in survival over time also appear more rapid in females than in males.
Table III. Trends in survival for cancer of the tongue by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Danish patients tend to have higher excess death rates during the first three months than the patients in the other countries (). The excess death rates have stayed fairly constant over time during the first year of follow-up (data not shown for 3–12 months of follow-up). In the subsequent years of follow-up, rates have been lower. There is a tendency towards decreasing survival with advancing age at diagnosis ().
Figure 4. Trends in age-standardised (ICSS) excess death rates per 100 person years for cancer of the tongue by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003. No Icelandic curves. Too few patients to calculate rates for Iceland.
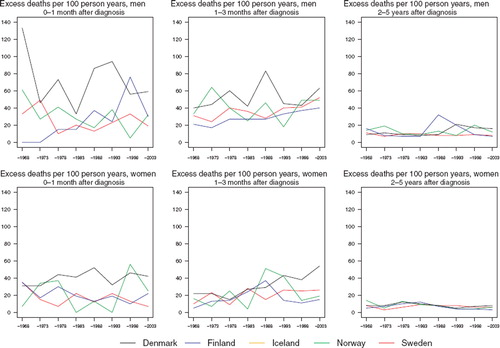
Table IV. Trends in 5-year age-specific relative survival in percent after cancer of the tongue by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the salivary glands
Incidence and mortality. The incidence rates in both sexes have stayed fairly constant over time in the last two decades, and there has been very little variation between countries (). The age-standardised incidence rates have been around 0.6 per 100 000 in males and 0.5 in females. Before 1980, a steep decline occurred in the rates in Denmark. The mortality rates conveyed a slightly decreasing trend with little systematic variation between countries, particularly among females ().
Figure 5. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for cancer of the salivary glands by sex and country. Nordic cancer survival study 1964–2003.
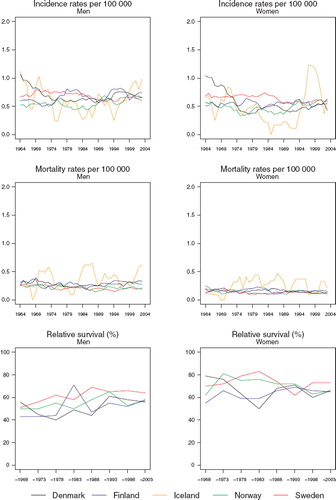
Survival. In males, the Swedish age-standardised 5-year relative survival ratios are somewhat higher than those in the other Nordic countries over the last two decades (, ). There seems to be a slight upward tendency in the ratios in males, but overall they still have remained lower than those of females. The small frequencies in Iceland do not permit calculation of relative survival.
Table V. Trends in survival for cancer of the salivary glands by sex and country. Number of tumours (N) included and the 5-year agestandardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
There is a lot of random variation in the excess death rates, and no systematic differences between countries can be seen (). The relative survival ratios are high in the youngest group, although they decline with advancing age at diagnosis ().
Figure 6. Trends in age-standardised (ICSS) excess death rates per 100 person years for cancer of the salivary glands by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003. No Icelandic curves. Too few patients to calculate rates for Iceland.
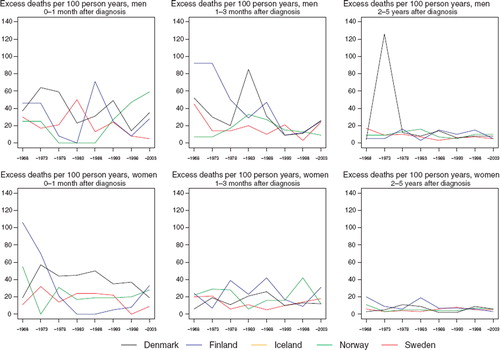
Table VI. Trends in 5-year age-specific relative survival in percent after cancer of the salivary glands by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the mouth
Incidence and mortality. Denmark has over time become the country with the highest incidence, with rates in men twice as high as those observed in the other Nordic countries (). The temporal development has been quite heterogeneous in males between the countries. The increase in Denmark seems to have attenuated recently. In Norway and Sweden, the rates have been decreasing over the period 1994–2003. In Finland, the development is similar to Denmark, although in absolute terms, rates are at a much lower level, while rates in Iceland have not been substantively altered over the last 20 years. Trends are similar in women, although the high incidence in Iceland is comparable to that among Danish women, with incidence and mortality rates similar between the sexes. The mortality rates display comparable patterns to the incidence rates but at a considerably lower level ().
Figure 7. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for cancer of the mouth by sex and country. Nordic cancer survival study 1964–2003.
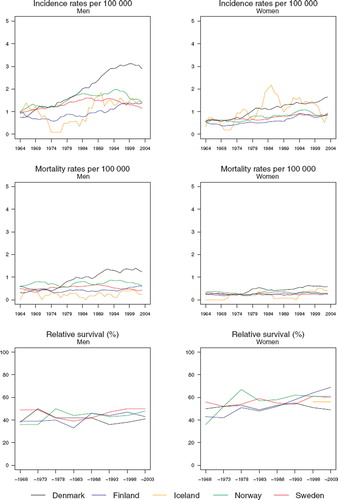
Survival. The Danish age-standardised 5-year relative survival ratios have during the most recent years not shown the increasing trends observed in the ratios for Finland, Norway and Sweden (, ). Overall, the ratios in females have tended to increase more rapidly with time than those for males.
Table VII. Trends in survival for cancer of the mouth by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
The excess death rates of the patients have by and large decreased in the first month (although there is a large degree of random variation) and in females 2–5 years after diagnosis in addition (). There is no discernible decline in excess mortality during the second and third month after diagnosis. There is however a tendency for relative survival to decrease with advancing age at diagnosis ().
Figure 8. Trends in age-standardised (ICSS) excess death rates per 100 person years for cancer of the mouth by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003.
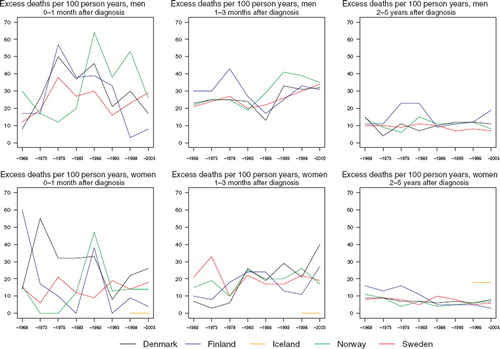
Table VIII. Trends in 5-year age-specific relative survival in percent after cancer of the mouth by sex and country. Nordic cancer survival study 1964–2003.
Cancer of the pharynx
Incidence and mortality. The incidence has increased sharply in Denmark, and to a lesser extent in Norway and Sweden, while rates are reasonably stable in Iceland and Finland (). The age-standardised incidence rates in 1999–2003 ranged in males from 1.7 in Finland and Iceland to 4.7 per 100 000 in Denmark. In females, rates range from 0.5 in Finland to 1.5 per 100 000 in Denmark. Mortality has been increasing only in Denmark, whereas rates have remained fairly stable during the last decades in the other countries ().
Figure 9. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for pharyngeal cancer by sex and country. Nordic cancer survival study 1964–2003.
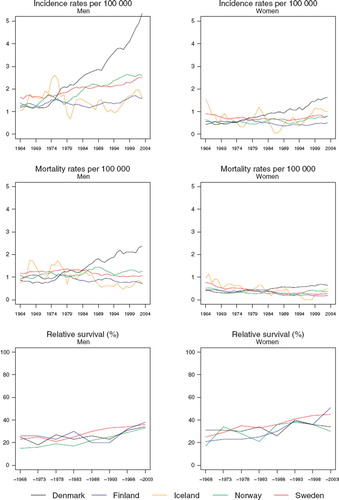
Survival. The age-standardised relative survival ratios have been steadily increasing over time in both sexes (, ). The survival of female patients has been higher than that of male patients. The differences between the countries were fairly unsystematic but the ratios in Sweden have had a tendency to be slightly higher than the others. No ratios from Iceland can be reported due to the small numbers of cases.
Table IX. Trends in survival for pharyngeal cancer by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
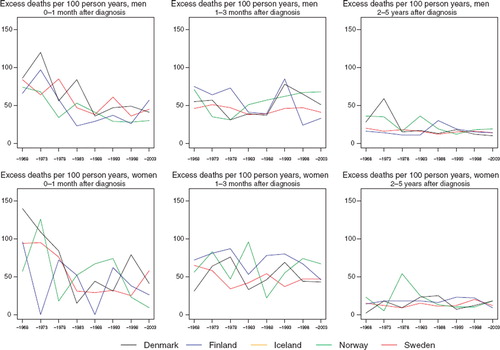
The excess death rates of the patients have been the highest in the first month after diagnosis, where they have decreased markedly with time (). No such decreases are observable in the other follow-up periods studied.
Figure 10. Trends in age-standardised (ICSS) excess death rates per 100 person years for pharyngeal cancer by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003. No Icelandic curves. Too few patients to calculate rates for Iceland.
The relative survival tends to be highest among the youngest patients (). There have been increments in survival in all age groups and countries, although in some age groups this cannot be documented because of the small number of cases.
Table X. Trends in 5-year age-specific relative survival in percent after pharyngeal cancer by sex and country. Nordic cancer survival study 1964–2003.
Discussion
The survival of patients following a diagnosis of lip cancer is very high with a rather limited potential for further improvement in prognosis. There are few survival differences by country, sex, age, or time period. As well as solar radiation, smoking is a known risk factor for lip cancer and may cause some co-morbidity resulting in an excess risk of death among patients. The Icelandic figures should be interpreted with caution due to the extremely small numbers involved.
The improvement in tongue cancer survival has been rather modest. The recent incidence peak in Denmark is not apparent in the relative survival even though the latter has been obtained by the hybrid method yielding the most up-to-date results. Continued follow-up will reveal whether the increase in incidence is accompanied by an increase in relative survival in Denmark, given the less dramatic changes in mortality.
There has been very little improvement in relative survival of patients with cancer of the salivary glands with time, and the differences between countries are small. The trends may also be affected by coding practices; the clinical and morphological evaluation of mixed tumours (pleomorphic adenomas), their reporting and coding at the Finnish Cancer Registry have, for example, changed over time [Citation8].
The stability of the relative survival ratios suggests that the increases in mouth and pharyngeal cancer incidence in Denmark are real and not primarily the result of differences in definitions or coding. The oral tumours form a heterogeneous group that may complicate comparisons. Smoking and alcohol are risk factors for oral cancers that may also have an effect on co-morbidity in a synergistic way, thus lowering the relative survival of the patients. Thus far, the higher consumption of oral tobacco in Sweden than in the other Nordic countries [Citation9,Citation10] is neither reflected in the incidence rates nor the relative survival ratios of the patients.
Many recent advances in therapy together with new standards of care [Citation11] are likely to play an important role in the observed increases in relative survival also in the Nordic countries. Also of particular clinical importance is the prognostic significance of the role of the HPV infection in squamous cell carcinomas of the oropharynx [Citation3]. The HPV-infected patients have a better prognosis than those not infected [Citation12].
Overall, patient survival has improved moderately in the Nordic countries. A likely continuation of the improvement may be expected with better targeted therapies. A lowering of risk and co-morbidity via primary prevention strategies that reduce the prevalence of smoking and alcohol in the Nordic populations presents a further challenge.
Acknowledgement
The Nordic Cancer Union (NCU) had financially supported the development of the NORDCAN database and program, as well as the survival analyses in this project.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Engholm G, Ferlay J, Christensen N, Bray F, Klint Å, Ólafsdóttir E, . (2008). NORDCAN: Cancer incidence, mortality and prevalence in the Nordic countries, version 3.3. Association of Nordic Cancer Registries. Danish Cancer Society (http://www.ancr.nu). Accessed 24 April 2009.
- Mayne ST, Morse DE, Winn DM. Cancers of the oral cavity and pharynx. Schottenfeld D, Fraumeni JF Jr. Cancer epidemiology and prevention. 3rd. Oxford: Oxford University Press; 2006. 674–96.
- Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol 2006; 24:2606–11.
- Berrino F, Capocaccia R, Coleman MP, Estève J, Gatta G, Hakulinen T, . Survival of cancer patients in Europe: the EUROCARE-3 Study. Ann Oncol 2003;14: Suppl 5.
- Verdecchia A, Guzzinati S, Francisci S, De Angelis R, Bray F, Allemani C, . Survival trends in European cancer patients diagnosed in 1988 to 1999. Eur J Cancer 2009; 45:1042–66.
- Engholm G, Gislum M, Bray F, Hakulinen T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 and followed up to the end of 2006. Material and methods. Acta Oncol 2010;49:545–60
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Dickman PW, Hakulinen T, Luostarinen T, Pukkala E, Sankila R, Söderman B, . Survival of cancer patients in Finland 1955–1994. Acta Oncol 1999;38:Suppl. 12.
- Dreyer L, Winther JF, Pukkala E, Andersen A. Tobacco smoking. Olsen JH, Andersen A, Dreyer L, Pukkala E, Tryggvadottir L, Gerhardsson de Verdier M, . Avoidable cancers in the Nordic countries. APMIS 1997;105:Suppl 76:9–47.
- Brennan P, Mucci L, Adami HO. Oral and pharyngeal cancer. Adami HO, Hunter D, Trichopoulos D. Textbook of cancer epidemiology. 2nd. Oxford: Oxford University Press; 2008. 155–74.
- Forastiere AA, Trotti A, Pfister DG, Grandis JR. Head and neck cancer: Recent advances and new standards of care. J Clin Oncol 2006;24:2603–5.
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, . Improved survival of patients with human papillomavirus – positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–9.