Abstract
Background. International comparisons have pointed to very low survival of patients diagnosed with testicular cancer (TC) in Estonia. Methods. Using population based data from the Estonian Cancer Registry and period analysis, we examined trends in TC survival between 1985 and 2004. Additional results from a review of clinical records to ascertain patterns of disease management (1990–2003) were used to explain the changes and identify the areas for potential improvement. Results. Age-adjusted 5-year period relative survival increased from 47.9% in 1985–1989 to 74.5% in 2000–2004 (p for trend <0.01). A marked improvement was seen for the patients younger than 30, with the 5-year survival reaching 93.3%, while the improvement remained modest among patients aged 30 and above. Although substantial advances occurred in staging and treatment techniques since 1990, deficiencies remained evident in disease management, including not referring patients to an oncologist after their orchiectomy and less careful diagnostic workup for patients above 30 years of age. Low use of radiotherapy suggests poor access to contemporary equipment. Delays in seeking medical consultation, but also in starting adjuvant therapy, could have contributed to poorer outcomes. Conclusions. Survival in TC increased markedly in Estonia by the 21st century, but is still notably lower than in the more developed countries. Multidisciplinary efforts may help to achieve further improvement. The provision of TC care should be coordinated by specialised cancer centres.
Testicular cancer (TC) is among the most curable malignancies today with 5-year survival over 90% in most of European countries [Citation1]. There is no effective primary prevention for TC, but timely diagnosis, adequate staging and treatment can provide an excellent prognosis.
Based on the results of the EUROCARE-3 project, striking differences in TC survival were observed across Europe, with Estonia standing out for its exceptionally low 5-year relative survival in 1990–1994 (71%) [Citation2].
While the incidence of TC, with an age-standardised (world) incidence rate of 2.2 per 100 000 men-years in 2002 is low in Estonia compared to the Nordic and Western European countries [Citation3], mortality is relatively high and decline in mortality was shown to be less marked in Estonia than in these countries [Citation4].
This paper aims to analyse recent trends in survival and management of TC in Estonia, a country formerly a part of the Soviet Union until regaining its independence in 1991, which was followed by transition to an open-market economy and an insurance-based health care system [Citation5]. This study is associated to the EUNICE-Survival cooperation, and relied on the data analysis resources described in detail elsewhere [Citation6].
Patients and methods
The survival analysis is based on the data from the Estonian Cancer Registry, which is population based and covers the whole country (territory 45 216 km2, population 1.34 million on 1 January 2008). Incident cases of TC diagnosed in 1980–2003 were included in the survival study, and the vital status of the patients was checked against the Estonian Population Registry on 31 December 2004.
Period analysis was used for examining trends in survival [Citation7]. Relative survival estimates were calculated as a ratio of the observed survival of the patients and the expected survival probabilities of the underlying general population [Citation8]. These probabilities were based on the population life-tables from Statistics Estonia. Model-based analysis, introduced by Brenner and Hakulinen [Citation9] was carried out: period-specific 5-year relative survival estimates were derived using a saturated model and modelling was used to derive a test for trend in survival across periods (between 1985–1989 and 2000–2004). For the period 2000–2004, with incidence data up to 2003 and follow-up data until 2004, hybrid analysis [Citation10,Citation11] was used, in order to enable the estimation of up-to-date survival in a situation where mortality data are more up-to-date than incidence data.
Survival estimates and p-values for survival trends are presented for all patients together and by three different age groups (15–29, 30–44 and ≥45 years) and two main morphological groups (seminoma and non-seminoma). To ensure the comparability between the time periods of diagnosis, the overall survival estimates were age-adjusted using weights from the International Cancer Survival Standards (ICSS) proposed by Corazziari et al. [Citation12]. Standard errors of the survival estimates were calculated using the delta method and statistical tests were considered significant at p<0.05.
In order to describe the patterns and temporal changes in TC management, the clinical records of patients diagnosed with TC in 1990–2003 were retrospectively reviewed to collect additional information on clinical characteristics such as diagnostic and staging procedures, extent of disease and modes of treatment. Percentages of patients with selected characteristics and p-values of trends between 1990–1994 and 2000–2003 are presented. STATA 10.0 software was used to conduct a Wilcoxon test for trend [Citation13].
Results
Survival analysis covered 332 patients diagnosed with TC in Estonia in 1980–2003 (). About one third (34.3%) of patients were 15–29 years of age at their diagnosis, 39.2% of patients were 30–44 and 26.5% of patients were 45 or older. Seminoma was diagnosed among 53.3% of patients and non-seminoma among 41.3% of patients; the other histological types and unspecified cancers were diagnosed among 5.4% of patients. The median age of patients was notably higher for seminoma than for non-seminoma (38 and 28 years, respectively).
Table I. Number of patients diagnosed with testicular cancer in Estonia in five periods between 1980 and 2003 and included in survival analysis, by age group and tumour morphology.
Overall, survival improved statistically significantly between 1985–1989 and 2000–2004 (). Crude 5-year relative survival increased from 49.5 to 81.8% (p value for trend <0.01), while the age-adjusted 5-year period relative survival increased from 47.9 to 74.5% (p<0.01).
Table II. Five-year period relative survival point estimates with 95% confidence interval (CI) for patients diagnosed with testicular cancer in Estonia in four periods between 1985 and 2004, and p-values for trend between 1985–1989 and 2000–2004, by age group and tumour morphology.
There was a considerable variation in survival trends by age groups: in case of the youngest patients (aged 15–29), the estimated 5-year relative survival rose from 49.5 to 93.3% (p<0.01), while survival improved from 47.7 to 79.9% among patients aged 30–44 years (p=0.07) and no improvement occurred among patients aged 45 and older, with estimated 5-year relative survival remaining at 41.1% in 2000–2004. A marked increase in patient survival was seen for both of the morphological groups, but the improvement was more pronounced for non-seminoma than seminoma.
Medical records were reviewed for all of the 225 patients diagnosed with TC in 1990–2003. Overall, 50% of the patients had Stage I disease (tumour confined to testis); Stage II (regional lymphnode involvement) and Stage III (distant metastases) were diagnosed equally for 24% of patients; the stage of disease was not specified for 2% of patients. Seminoma was more often localised at diagnosis than non-seminoma (64% vs. 37% of the cases, respectively). presents the stage distribution of seminomatous and non-seminomatous tumours diagnosed among patients aged 15–29 and those aged 30 and above. In seminoma, breakdown by stage was similar within these two age groups, while in non-seminoma the distant metastases were diagnosed more often among older patients than among younger patients (36% vs. 23%, respectively). Changes in stage distribution were generally modest between 1990–1994 and 2000–2003, but a statistically significant rise was seen in the proportion of Stage II cases for the youngest patients diagnosed with non-seminoma.
Table III. Stage distribution of seminomatous and non-seminomatous testicular cancers diagnosed in Estonia in 1990–2003, by age group.
and present the percentages of patients identified with selected diagnostic and treatment characteristics in three periods between 1990 and 2003 and p-values of the trends between 1990–1994 and 2000–2003.
Table IV. Selected diagnostic characteristics among patients diagnosed with testicular cancer in Estonia in three periods between 1990 and 2003, and p-values for trend between 1990–1994 and 2000–2003.
Most of the patients had chest x-ray or computerised tomography (CT) (). Ultrasound sonography (USG) was performed for two of three patients in the 1990s. Abdominal and especially pelvic CT were very rare during the early 1990s, but in 2000–2003, these imaging procedures were performed for all patients aged 15–29 and about 80% of patients aged 30 and above, while the use of USG decreased.
The use of prognostic serum markers was low in 1990–1994 with alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) examined only for one of 10 patients. Marker use has rapidly increased since the mid-1990s, especially for the youngest patients. By 2000–2003, AFP and HCG were examined for 97% of patients aged 15–29 years and 74% of patients aged 30 years and over, while lactate dehydrogenase (LDH) was examined for 85% and 68% of patients, respectively.
Inguinal orchiectomy was a primary treatment for 96% of the patients. Surgery was performed mainly at the urology departments of general hospitals and specific cancer therapies were given at the two specialised cancer centres where patients were referred to after their surgery. The median time interval between orchiectomy and beginning of adjuvant therapy was three weeks, whereas among 8% of patients the time lag exceeded two months. Overall, 6% of patients (most of them were above 30 years of age and diagnosed with seminoma) were not referred to the cancer centres, suggesting that these patients may not have been seen by an oncologist.
The proportion of patients who underwent retroperitoneal lymphnode dissection (RPLND) increased from 21% in 1990–1994 to 43% in 2000–2003 (p<0.01) (). On the other hand, deficiencies were evident in completeness of information in pathology reports: the number of discovered lypmhnode metastases was not recorded for 39% of patients and the number of examined nodes was not recorded for 64% of patients who underwent RPLND. For the cases with this information present in pathology reports, the total number of examined nodes varied from 1 to 16 (the median number of examined nodes was 2).
Table V. Selected treatment characteristics among patients diagnosed with testicular cancer in Estonia in three periods between 1990 and 2003, and p-values for trend between 1990–1994 and 2000–2003.
Overall, radiotherapy was applied to 51% of patients with seminoma and 6% of patients with non-seminoma; chemotherapy was given to 43% of patients with seminoma and 83% of patients with non-seminoma. Both radiotherapy and chemotherapy were given to 16% of patients. Between 1990–1994 and 2000–2003, the proportion of patients receiving radiotherapy for seminoma remained rather stable, while there was a clear increase in proportion of patients having undergone chemotherapy for non-seminoma (see ). Among the patients receiving chemotherapy, the use of platinum-based schemes increased from 78 to 98% (p=0.01).
Discussion
Despite unabated increases in TC incidence, a marked decline in mortality has been observed in several European countries already since the mid-1970s, due to advances in chemotherapy and best-practice tumour management [Citation4]. Nevertheless, in several lower-resource countries mortality rates remain relatively high even since 2000 [Citation3].
Comparative data on cancer survival and its trends in Europe has become increasingly available over the past 20 years, thanks to the co-operative efforts of population-based cancer registries [Citation1,Citation2,Citation6,Citation14]. Wide variation in TC survival has been revealed within Europe, but the discrepancies were also evident among the former Eastern Block countries that have provided data for these comparisons. For example, the estimated 5-year relative survival was approaching 90% in Slovenia in 1990–1994, while remaining under 60% in Poland (Cracow) and Estonia (see ). By 2000–2004, survival in Slovenia reached a level similar to the Nordic countries (93–94%), and, considering the low starting point, the improvement in survival was faster in Poland than Estonia. We can assume that, similar to Estonia, the progress could have been slower than expected also in some other Eastern European countries which have not been included in survival comparisons, but where reductions in mortality have been modest.
Table VI. Trends in mortality and survival in testicular cancer in selected European countries between 1990–1994 and 2000–2004.
In the long run, we observed a clear upward trend in TC survival in Estonia: since the late 1980s the age-adjusted 5-year relative survival has increased from 47.9 to 74.5%. Despite this major improvement the 5-year relative survival estimate for the period of 2000–2004 still remained considerably lower in Estonia than the cohort-based rates observed in most other European countries already one or two decades before [Citation2]. Furthermore, in another analysis using model-based projection, the age-adjusted 5-year relative survival in Estonia during 2005–2009 was projected to be of 81.8%, which was the lowest estimated survival among the 11 populations included in the EUNICE survival comparison [Citation14].
A marked improvement in survival was observed for the youngest patients (aged 15–29), and by 2000–2004, their estimated 5-year relative survival in Estonia nearly approached the average European level reported by EUROCARE-4 project [Citation1]. In contrast, the estimated survival remained still low for the age group 30–44, and markedly lower for the age group of 45 and older. Thus, a further improvement in TC survival in Estonia appears possible only when an improvement is achieved among patients above 30 years of age.
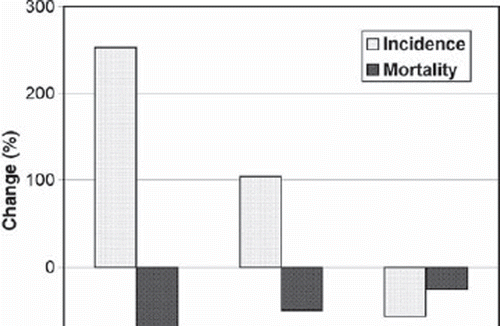
The incidence and mortality data support our findings regarding the differences in survival trends by the age group (see ). Between 1980–1984 and 2000–2004, a dramatic rise in the age-standardised incidence rates (253%), but also a major decline in the age-standardised mortality rates (–70%) have occurred in Estonia for the age group 15–29, while the extent of changes has been smaller among men aged 30–44 and especially among those aged 45 and above.
Figure 1. Percentage change in the age-standardised (world) incidence and mortality for testicular cancer in Estonia between 1980–1984 and 2000–2004, by the age group. The incidence is based on the Estonian Cancer Registry; the mortality is based on the Estonian Cancer Registry (1980–1984) and the World Health Organisation Cancer Mondial Information System, http://www-dep.iarc.fr (2000–2004).
The great disparities observed in TC survival in Estonia in relation to patients’ age are rather exceptional in the international context. Given that TC patients are much younger on average than those with most other malignancies, their treatment is usually not prevented by age-related health problems. According to the EUROCARE-4 comparison, the area-weighted 5-year relative survival rate for patients aged 45–54 was only slightly lower than for younger patients (94% and 95%, respectively), and a certain decrease in survival was observed only after the age of 65 [Citation1]. In another large-scale study based on SEER data, a poorer stage- and morphology-adjusted survival was also found for patients older than 50, particularly in metastasised disease [Citation15]. Lower tolerance to chemotherapy and suboptimal treatment were mentioned among the possible reasons.
Although a clear improvement in use of prognostic markers (AFP, HCG and LDH) was seen since the mid-1990s, the markers were not examined for all patients in 2000–2003. Serum-based markers are helpful in several aspects of care, including appropriate diagnosis, prognosis and surveillance for recurrence [Citation16], and the under-use or failure to document the use of markers may indicate less than optimal care [Citation17]. We observed a notably lower use of imaging and markers for the older patients compared to the younger patients. Modern diagnostic techniques were introduced in Estonia in the mid-1990s, considerably later than in wealthy European countries [Citation5]. The lack of access to important staging tools [Citation18] but also low health care resources in Estonia could have influenced the diagnostic workup depending on the age of patients.
Distributions of patients by age at diagnosis and tumour morphology in Estonia are rather similar to those in other countries [Citation19], while the proportion of advanced cases (especially for non-seminoma) was found to be relatively high in Estonia [Citation20]. In our study, the proportion of Stage III cases in non-seminoma was considerably higher among patients aged 30 or above than those aged 15–29, suggesting a longer diagnostic delay among older patients. For very young patients diagnosed with non-seminoma, also a significant increase in proportion of Stage II disease was observed between 1990–1994 and 2000–2003, which could be partly related to migration from Stage I towards Stage II due to intensive use of staging procedures.
A notable increase in the number of patients treated by RPLND was observed through 1990–2003. This surgical procedure is an important staging tool to define subsequent treatment requirements, but also, it is shown to be curative in up to 90% of patients with low-volume retroperitoneal disease [Citation21]. According to our observations however, the number of examined lymphnodes was generally low, what could have contributed to under-staging and less than adequate treatment.
Platinum-based treatment was introduced in Estonia from the 1990s, and by 2000, cisplatin-containing schemes accounted for 98% of all chemotherapy given for TC. Despite the improved compliance with consensus guidelines [Citation16], the use of radiotherapy in seminoma remained relatively low, which might reflect poor availability of contemporary radiation equipment in Estonia until very recently. Furthermore, the review of clinical records in two Estonian cancer centres indicated that a number of patients diagnosed with seminoma have never been referred to an oncologist after their surgery. For the vast majority of patients with localised seminoma, surgery alone is curative indeed, but the standard management strategy in the 1990s has included adjuvant radiotherapy [Citation22] and only more recently, adjuvant chemotherapy or surveillance have acquired support as acceptable strategies for Stage I seminoma [Citation16]. Selection of an appropriate management to prevent relapse of disease or detect it early requires the skills of a multidisciplinary team, which can be best attained in specialised centres [Citation23]. In Estonia, a lack of collaboration between urologists and oncologists and not referring patients to cancer centres for adjuvant treatment could have contributed to the undue spread of disease and thus, to poorer long-term outcomes.
In conclusion, access to modern care and patient survival in TC has markedly improved in Estonia since 1990. Still, the estimated period survival remained relatively low in the beginning of the 21st century, especially for patients older than 30 at the time of their diagnosis. The study pointed to several deficiencies in disease management, including not referring patients to an oncologist after their orchiectomy and less careful diagnostic workup for older patients. Low use of radiotherapy suggests poor access to contemporary equipment until very recently. Concerted multidisciplinary efforts may help to reduce the gap in TC survival between Estonia and the more developed European countries. In a small country like Estonia, it would be adequate and possible to refer all the patients into the specialised cancer centres for appropriate staging, planning of risk-based treatment and surveillance. To reduce delays in patient presentation, much more attention should be paid to promoting health education.
Acknowledgements
We wish to thank Pille Härmaorg and Tatjana Veideman for their assistance in clinical data analysis. This work was supported in Estonia by the Estonian Science Foundation (grant no 6072). Performing survival analyses in international collaboration was partly supported by a grant from the European Commission (Directorate of SANCO, Luxemburg) for the European Network for Indicators on Cancer (EUNICE). There is no conflict of interest to be declared.
References
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, . EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, . EUROCARE-3: Survival of cancer patients diagnosed 1990–94 – results and commentary. Ann Oncol 2003;14(Suppl 5):v61–118.
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. IARC CancerBase No. 5. version 2.0, Lyon: IARCPress; 2004.
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Møller H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. Int J Cancer 2006;118:3099–111.
- Aareleid T, Brenner H. Trends in cancer patient survival in Estonia before and after the transition from a Soviet republic to an open market economy. Int J Cancer 2002;102:45–50.
- Gondos A, Bray F, Brewster DH, Coebergh JWW, Hakulinen T, Janssen-Heijnen MLG, . Recent trends in cancer survival across Europe between 2000 and 2004: A model-based period analysis from 12 cancer registries. Eur J Cancer 2008;44:1463–75.
- Brenner H, Gefeller O, Hakulinen T. Period analysis for ‘up-to-date’ cancer survival data: Theory, empirical evaluation, computational realisation and applications. Eur J Cancer 2004;40:326–35.
- Ederer F, Axtell LM, Cutler SJ. The relative survival rate: A statistical methodology. Natl Cancer Inst Monogr 1961;6:101–21.
- Brenner H, Hakulinen T. Up-to-date and precise estimates of cancer patient survival: Model-based period analysis. Am J Epid 2006;164:689–96.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Brenner H, Hakulinen T. Model based hybrid analysis of cancer patient survival. Eur J Cancer 2007;43:921–7.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer 2004;40:2307–16.
- Cuzick J. A Wilcoxon-type test for trend. Stat Med 1985; 4:87–90.
- Gondos A, Bray F, Hakulinen T, Brenner H, the EUNICE Survival Working Group. Trends in cancer survival in 11 European populations from 1990 to 2009: A model-based analysis. Ann Oncol 2009;20:564–73.
- Spermon JR, Witjes JA, Kiemeney LA. Difference in stage and morphology-adjusted survival between young and elderly patients with a testicular germ cell tumor. Urology 2002;60:889–93.
- Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, . Guidelines on testicular cancer. European Association of Urology 2009. Available from: http://www.uroweb.org/fileadmin/tx_eauguidelines/2009/Full/Testis_Cancer.pdf.
- Gilbert SM, Daignault S, Weizer AZ, Wei JT, Hollenbeck BK. The use of tumor markers in testis cancer in the United States: A potential quality issue. Urol Oncol 2008;26:153–7.
- Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. Am J Roentgenol 2008;191:387–95.
- Agnarsson BA, Gudbjartsson T, Einarsson GV, Magnusson K, Thoroddsen A, Bergthorsson JT, . Testicular germ cell tumours in Iceland: A nationwide clinicopathological study. APMIS 2006;114:779–83.
- Sant M, Aareleid T, Artioli ME, Berrino F, Coebergh JW, Colonna M, . Ten-year survival and risk of relapse for testicular cancer: A EUROCARE high resolution study. Eur J Cancer 2007;43:585–92.
- Stephenson AJ, Sheinfeld J. The role of retroperitoneal lymph node dissection in the management of testicular cancer. Urol Oncol 2004;22:225–33.
- Bernal F, Raman JD. Exploration of treatment options for the management of stage I testicular seminoma. Expert Rev Anticancer Ther 2008;8:1081–90.
- Huddart RA, Birtle AJ. Recent advances in the treatment of testicular cancer. Expert Rev Anticancer Ther 2005;5:123–38.