Abstract
Ibandronate, one of the most potent bisphosphonates, has been shown to inhibit growth of various cancer cell lines. In contrast, little is known about the effects of ibandronate on prostate cancer cells. Therefore the aim of our study was to characterize the effects of ibandronate alone and in combination with docetaxel on the growth of prostate cancer cell lines and to identify the underlying signalling pathways. Material and methods. The prostate cancer cell lines LNCaP and PC-3 were treated with increasing concentrations of ibandronate and docetaxel alone and in combination. Viable cell number was measured after five days using a hemocytometer and the MTT-method. The effects of ibandronate were tentatively antagonized by addition of farnesyl-pyrophosphate (FPP) or farnesol (FOH). Results. Ibandronate inhibits growth of both prostate cancer cell lines in a dose dependent manner. In combination with docetaxel, synergistic effects are found as evidenced by a combination index (CI) of <1. Addition of FOH and FPP completely antagonized the growth inhibitory effects of ibandronate on both cell lines. Surprisingly, in combination with ibandronate and docetaxel, FOH further increased growth inhibition instead of antagonizing the growth inhibitory effects of ibandronate. Furthermore, FOH alone appeared to be a potent inhibitor of tumor cell growth. Discussion. Ibandronate effectively inhibits growth of prostate cancer cell lines via inhibition of the farnesyl-IPP-synthase and exhibits synergistic effects with docetaxel. In addition, FOH is a potent inhibitor of prostate cancer cell lines and may display an interesting treatment option for patients with CRPC.
Along with taxanes, bisphosphonates represent the standard of care for patients with metastatic prostate or breast cancer for the prevention of skeletal-related events, including pathological fractures or the need for radiation or surgery to the bone [Citation1]. Bisphosphonates mainly function on osteoclasts or other bone cells in the immediate microenvironment of osteoclasts [Citation2]. However, they have also been reported to affect tumor cells in various cancer types including prostate cancer [Citation3–10]. Because of the simultaneous use of taxanes and bisphosphonates in cancer patients, their additive or synergistic effects are most interesting. Zoledronic acid and ibandronate (Iban), two of the most potent bisphosphonates, have already been shown to act synergistically with taxanes in breast cancer cells [Citation11,Citation12]. In prostate cancer cells, the combination of zoledronic acid and docetaxel has been shown to downregulate anti-apoptotic Bcl-2, resulting in the induction of apoptosis [Citation13]. The downregulation of Bcl-2 by zoledronic acid is a result of the inhibition of protein geranyl-geranylation [Citation11,Citation14], which subsequently, at least in breast cancer, leads to the inactivation of the Ras pathway, which regulates Bcl-2 expression [Citation15]. In prostate cancer cells, the action of zoledronic acid appears to be independent of membrane-bound ras [Citation16]. Most recently it has been shown that zoledronic acid leads to a decreased expression of the cysteine-rich angiogenic inducer 61 (CYR61) which was paralleled by the inhibition of Ras dependent signalling pathways [Citation17].
In contrast, little is known about the effects of ibandronate in prostate cancer, especially regarding the signalling pathways that may be involved in the action of ibandronate. For example, ibandronate has been reported to induce apoptosis and to inhibit growth in J774 macrophage-like cells. However, the addition of farnesol (FOH) or geranylgeraniol (GGOH) has been shown to reverse farnesylation and geranyl-geranylation, antagonizing the effects of ibandronate [Citation18]. To date, only a single study has examined the effects of ibandronate on the growth of the androgen-independent PC-3 cell line [Citation10]. Therefore, the role of ibandronate in the treatment of metastatic prostate cancer remains controversial [Citation19]. The aim of our study was to characterize the effects of ibandronate and docetaxel (Doc) alone and in combination on the growth of prostate cancer cells in vitro. Furthermore, the underlying signalling pathways focusing on the mevalonate pathway were studied in order to define the role of farnesylation and geranyl-geranylation for the effects induced by ibandronate.
Material and methods
Cell lines
The adherently growing prostate carcinoma cell lines LNCaP and PC-3, obtained from the DSMZ (Braunschweig, Germany), were routinely cultured in RPMI (Sigma-Aldrich, Munich, Germany) supplemented with 10% foetal calf serum, L-Glutamine and gentamicin. Cells were grown in a humidified atmosphere of 5% CO2 at 37°C and sub-cultured using 1 nM EDTA solution (Sigma-Aldrich, Munich, Germany).
Drugs
Ibandronate was a kind gift from Roche Diagnostics GmbH (Penzberg, Germany). Docetaxel (Doc), farnesol (FOH) and farnesylpyrophosphate (FPP) were purchased from Sigma-Aldrich (Munich, Germany). Stock solutions of ibandronate and farnesol in phosphate buffered saline (PBS) and docetaxel in dimethylsulfoxide (DMSO) were prepared. Before using the drugs, the stock solutions were diluted to the working concentrations in cell culture media.
Cell viability assay
Cells were seeded at 104 cells/100 μl in 96-well plates and incubated at 37°C overnight to allow adherence to the plate. After removal of the growth media, the cells were treated with increasing concentrations of either drug. Control cultures contained the same concentration of the diluting substance (PBS or DMSO) comparable to cells that were treated with the maximum concentrations of ibandronate or docetaxel. On day 5, the cell number was measured using a haemocytometer and the MTT method as previously described [Citation20]. In brief, the culture medium was removed and the cells were washed with PBS. Then, 50 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich, Munich, Germany) was added to each well at a final concentration of 0.5 mg/ml. The cells were further incubated for 4 h at 37°C in a humidified 5% CO2 atmosphere to allow the formation of formazan crystals in metabolically active cells. After incubation, the MTT solution was removed, and the cells were washed with DMSO in order to solubilise the formazan crystals. The absorbance of the each cell lysate was measured at 570 nm. The results are expressed as a percentage (%) of treated versus untreated cells.
Statistical analysis
All experiments were performed at least three times. Data for the cell viability assays represent the means of the results obtained for quadruplicate samples in a representative experiment. The significance of the results was determined by a 2-tailed Student's t-test using the software Sigma Plot 9.0 (SPSS Science, Chicago, IL). A p-value <0.05 was considered as significant.
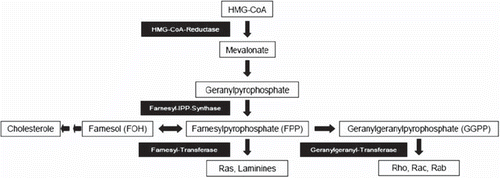
The combined effects of the drugs in terms of synergism, summation, or antagonism were quantitatively analyzed by the median effect plot derived by Chou [Citation21] as follows: fa/fu = (D/Dm)m, where fa indicates the response, fu unresponsiveness, D the dosage, Dm the dosage of half inhibition, m the slope and fu = 1 – fa. The interaction of 2 drugs is quantitatively determined by the combination index (CI), which is calculated as follows: CI = D1/Dx1 + D2/Dx2 + (α*D1*D2)/(Dx1*Dx2), where DX is the dose that is required to produce x% inhibition. When CI = 1, summation is indicated; when CI < 1, synergism is indicated; when CI < 1, antagonism is indicated. The effects of the two drugs are mutually nonexclusive (α = 1).
Results
Effects of Ibandronate on LNCaP and PC-3 cell growth
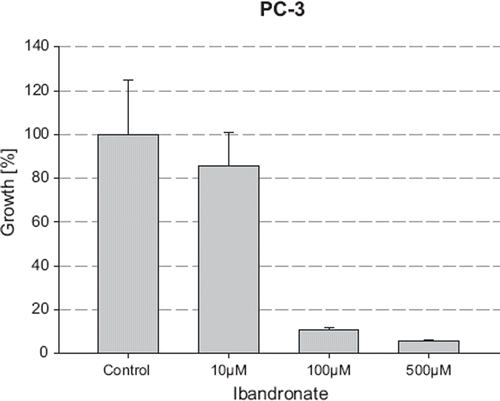
Treatment of LNCaP and PC-3 cells for five days with increasing concentrations of ibandronate caused a dose-dependent inhibition of cell growth, as measured by both a haemocytometer (data not shown) and the MTT assay (). Compared to the coulter method, the MTT assay was more sensitive for measuring the growth inhibitory effects of ibandronate treatment. Using the MTT method, ibandronate reduced the number of viable LNCaP cells from 16.67% at 10 μM to 0.45% at 100 μM, compared to the controls (p<0.05), resulting in an IC50 value of 3.7 μM. PC-3 cells showed a lower sensitivity to ibandronate with a decrease in cell viability to 80.37% at 10 μM and 10.89% at 100 μM with a calculated IC50-value of 33.69 μM ().
Figure 1. Growth inhibition of LNCaP cells by ibandronate. The cell number was measured after incubation with increasing concentrations of ibandronate for five days using the MTT assay. Values represent means ± S.D., n=4. Significant difference *=p<0.05; **=p<0.01 between two survival fractions of treated cells and control cells.
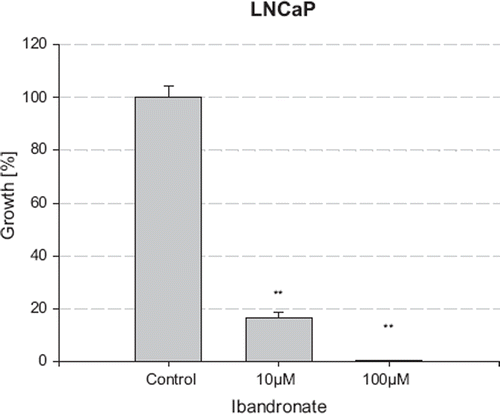
Figure 2. Growth inhibition of PC3 cells by ibandronate. The cell number was measured after incubation with increasing concentrations of ibandronate for five days using the MTT assay. Values represent means ± S.D., n=4. Significant difference *=p<0.05; **=p<0.01 between two survival fractions of treated cells and control cells.
Effects of Docetaxel on LNCaP and PC-3 cell growth
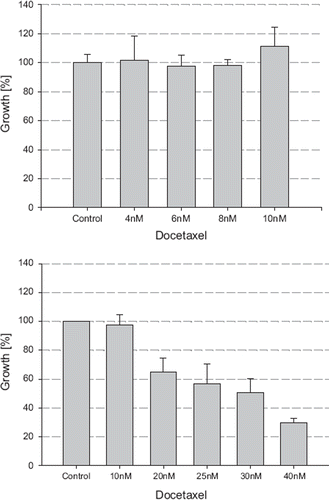
Incubation of LNCaP and PC-3 cells with increasing concentrations of docetaxel (4 nM–40 nM) led to a dose-dependent decrease in the number of viable cells, ranging from 0% at 4 nM to 29.69% and 7.84% at 40 nM in LNCaP and PC-3 cells, respectively (). The corresponding IC50 values were calculated as 24 nM and 13 nM, respectively. The concentration of 4 nM docetaxel, which demonstrated no evidence of growth inhibitory or stimulatory effects on prostate cancer cells, was then chosen to investigate the combinatory effects of ibandronate and docetaxel.
Figure 3. Growth inhibition of LNCaP cells by docetaxel. The cell number was measured after incubation with increasing concentrations of docetaxel for five days using the MTT assay. Values represent means ± S.D., n=4. Significant difference *=p<0.05; **=p<0.01 between two survival fractions of treated cells and control cells.
Effects of Ibandronate in combination with Docetaxel on LNCaP and PC-3 cell growth
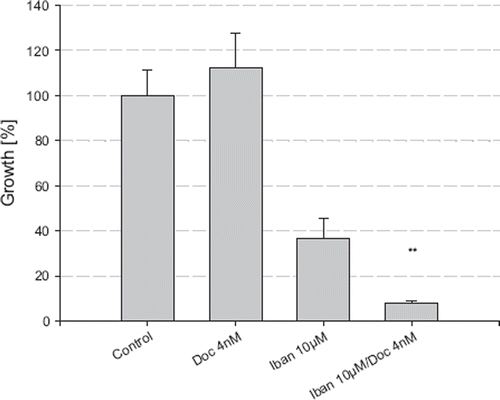
The combination of ibandronate (10 μM) and docetaxel (4 nM) showed greater growth inhibitory effects on LNCaP cells compared to ibandronate (10 μM) or docetaxel (4 nM) treatment alone, reducing the cell number to 8.05% compared to the control (p<0.05) (). Using the median effect plot method and calculation of the combinatory index (CI) revealed synergistic effects of the combination of ibandronate and docetaxel, with a CI=0.659 (data not shown). Comparable synergistic effects were found in PC-3 cells (CI=0.76, data not shown).
Figure 4. Combination of Ibandronate and docetaxel in LNCaP. The cell number was measured after incubation with ibandronate and/or docetaxel for five days using the MTT assay. Values represent means ± S.D., n=4. Significant difference *=p<0.05; **=p<0.01 between two survival fractions of treated cells and control cells.
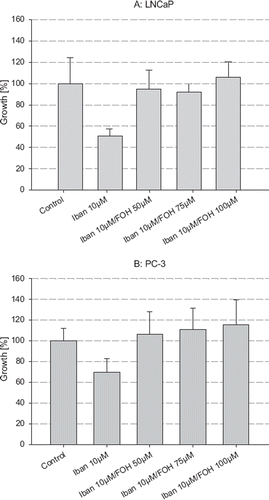
Reversibility of growth inhibition of ibandronate by Farnesol (FOH) and Farnesylpyrophosphate
The addition of FOH at concentrations of 50, 75 and 100 μM was able to completely antagonize the growth inhibitory effects of ibandronate in both LNCaP and PC-3 cells (). Furthermore, the addition of increasing concentrations of farnesylpyrophosphate (FPP), ranging from 1 μM to 10 μM, were able to revert the growth inhibition of ibandronate in both cell lines (data not shown).
Figure 5. Reversibility of growth inhibition of ibandronate by addition of farnesol (FOH). The cell number of LNCaP (A) and PC-3 (B) cells was measured after incubation with ibandronate alone and in combination with increasing concentrations of FOH for five days. Values represent means ± S.D., n=4. Significant difference *=p<0.05; **=p<0.01 between two survival fractions of treated cells and control cells.
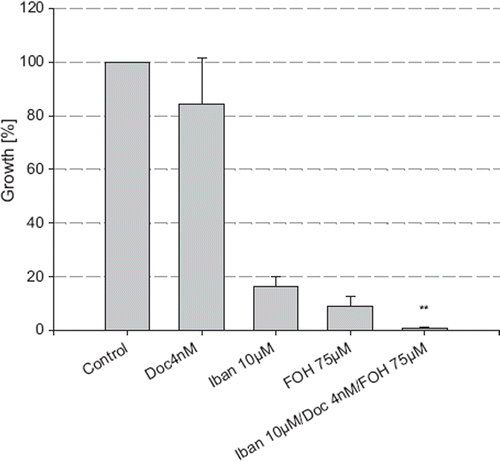
Effects of farnesol alone and in combination with ibandronate and docetaxel
In combination with docetaxel (4 nM) and ibandronate (10 μM), FOH (75 μM) was not able to reverse the growth inhibitory effects of ibandronate but instead enhanced the growth inhibition (). Surprisingly, FOH (75 μM) treatment alone demonstrated strong growth inhibitory effects by reducing the cell number to 8.8% compared to the control (p<0.05).
Figure 6. Growth inhibition of LNCaP by combination treatment of ibandronate, docetaxel and FOH. The cell number was measured after incubation with ibandronate, docetaxel and farnesol alone and in combination for five days, using the MTT assay. Values represent means ± S.D., n=4. Significant difference *=p<0.05; **=p<0.01 between two survival fractions of treated cells and control cells.
Discussion
Bisphosphonates have been used along with taxanes for several years for the treatment of patients with breast and prostate cancer. Of these, zoledronic acid has been extensively studied to unravel its underlying mechanism of action on tumor cells. Nevertheless, the potentials of zoledronic acid for cancer treatment is still underestimated and needs to be further studied [Citation22]. In addition to monotherapy, additive or synergistic effects of zoledronic acid in combination with taxanes have been found in breast and prostate cancer tumor cell lines [Citation10–12]. Ibandronate, a second-generation bisphosphonate, has only been approved for the prevention of skeletal-related events in breast cancer and without restriction for the treatment of tumor-induced hypercalcaemia by the European Medicines Agency. Due to its favorable toxicity profile and efficacy in the palliation of metastatic bone pain, ibandronate may represent another candidate for the treatment of metastatic prostate cancer [Citation19]. In contrast to zoledronic acid, little is known about the effects of ibandronate on prostate cancer cells and its underlying mechanism. The results of the present study demonstrate a dose-dependent growth inhibition of the prostate cancer cell lines PC-3 and LNCaP by ibandronate. These results contribute to the results of a former study on the effects of different bisphosphonates, including ibandronate, on the growth of PC-3 cells [Citation10]. Ibandronate therefore, apart from its effects on osteoclasts and osteoblasts, exerts direct effects on both hormone-sensitive and castration-resistant prostate cancer cells. In addition, ibandronate has been shown to inhibit tumor cell invasion and adhesion of PC-3 cells to bone [Citation23,Citation24]. Taken together these results encourage to investigate the clinical use of ibandronate for the treatment of patients with prostate cancer that has metastasized to the bone.
Inhibition of the mevalonate pathway and the subsequent prenylation of proteins have been identified as the main mechanism of action of bisphosphonates for the inhibition of tumor cell growth (). Given the fact that the effects of bisphosphonates may differ among different bisphosphonates and cell lines [Citation25], further studies were directed to investigate the role of farnesylation and geranyl-geranylation for the effects of ibandronate. Our results showed that the addition of farnesol (FOH) or farnesylpyrophosphate (FPP) to ibandronate was able to completely antagonize the growth inhibitory effects of ibandronate on the growth of PC-3 and LNCaP cells. Comparable results have been found in the murine macrophage cell line J774, where the addition of farnesol was able to inhibit the activation of caspase-3 and the induction of apoptosis by ibandronate [Citation18]. Moreover, it has been shown that ibandronate inhibits the prenylation of Ras through the inhibition of farnesyltransferase [Citation26]. Along with the published literature our results stress that the growth inhibition of prostate cancer cell lines by ibandronate is caused by the prevention of protein farnesylation via inhibition of the farnesyl-IPP-synthase (). In contrast, prevention of geranyl-geranylation has been identified as the main way of action of zoledronic acid to cause growth inhibition of prostate cancer cells. Addition of geraniol but not farnesol was able to completely antagonize the growth inhibitory effects by zoledronic acid [Citation14,Citation17,Citation27].
Since bisphosphonates are used along with docetaxel in prostate cancer patients, we aimed to detect possible additive or even synergistic effects of ibandronate and docetaxel. Synergistic effects of zoledronic acid and docetaxel have already been shown in prostate cancer cell lines [Citation28,Citation29]. Concerning ibandronate, synergistic effects with taxanes have only been demonstrated for the invasion of breast cancer cells and their adhesion to bone [Citation12]. No synergy was found for the induction of apoptosis. As a result, the authors concluded that the synergistic effects of ibandronate and taxanes were independent on direct effects on tumor cells and affected only the microenvironment of the osteoclasts. In contrast, the results of our studies revealed synergistic effects of ibandronate and docetaxel on the growth of prostate cancer cell lines. Therefore, synergism seems not to be restricted to the microenvironment of cancer cells. Since both, bisphosphonates and taxanes, independently regulate the expression and function of Bcl-2, synergism may be caused, at least in part, by their ability to induce apoptosis via regulation of Bcl-2 [Citation15,Citation30,Citation31]. Antagonizing the effects of either docetaxel or ibandronate should be able to abrogate synergism. Surprisingly, the addition of farnesol in our studies did not lead to a reduction of the growth inhibition but a further decrease in the viability of the cells.
Growth inhibition and the induction of apoptosis by farnesol has been shown for several benign and malignant cells [Citation30,Citation32,Citation33] and we were able to demonstrate, for the first time, the growth inhibitory effects of farnesol in prostate cancer cell lines. Apart from the restoration of farnesylation in the presence of ibandronate, farnesol may exert effects that are independent from the mevalonate pathway that lead to the additional growth inhibitory effects in combination with ibandronate and docetaxel. These effects may also, at least in part, be explained by the ability of farnesol to induce apoptosis by either induction of endoplasmatic reticulum (ER) stress and/or its effects on cytosolic diacylglycerol (DAG) which regulates phosphorylation of Bcl-2 [Citation34–36].
In summary, our results demonstrated, for the first time, a dose-dependent growth inhibition of androgen-dependent and androgen-independent prostate cancer cell lines by ibandronate. The growth inhibition appeared to be caused by the prevention of protein prenylation via the inhibition of the farnesyl-IPP-transferase. Furthermore synergistic effects of ibandronate and docetaxel have been found which may have clinical relevance for the treatment of patients with metastatic prostate cancer. Surprisingly, farnesol, when used alone and in combination with ibandronate and docetaxel, was found to be a potent growth inhibitor of prostate cancer cell lines. The interaction of ibandronate, farnesol and docetaxel may be linked by the effects on the expression and function of Bcl-2, a major regulator of apoptosis. Further investigations are underway to reveal the underlying mechanism for the synergistic effects of ibandronate, farnesol and docetaxel and to define the role of Bcl-2 in this context.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, . EAU guidelines on prostate cancer. Eur Urol 2008;53:68–80.
- Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, . Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000;88:2961–78.
- Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000;82:1459–68.
- Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer 2001;84: 951–8.
- Aparicio A, Gardner A, Tu Y, Savage A, Berenson J, Lichtenstein A. In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 1998;12:220–9.
- Riebeling C, Forsea AM, Raisova M, Orfanos CE, Geilen CC. The bisphosphonate pamidronate induces apoptosis in human melanoma cells in vitro. Br J Cancer 2002;87: 366–71.
- Lee MV, Fong EM, Singer FR, Guenette RS. Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res 2001;61:2602–8.
- Tassone P, Tagliaferri P, Viscomi C, Palmieri C, Caraglia M, D'Alessandro A, . Zoledronic acid induces antiproliferative and apoptotic effects in human pancreatic cancer cells in vitro. Br J Cancer 2003;88:1971–8.
- Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 2000;15:2211–21.
- Dumon JC, Journe F, Kheddoumi N, Lagneaux L, Body JJ. Cytostatic and apoptotic effects of bisphosphonates on prostate cancer cells. Eur Urol 2004;45:521–8.
- Jagdev SP, Coleman RE, Shipman CM, Rostami H, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: Evidence for synergy with paclitaxel. Br J Cancer 2001;20:1126–34.
- Magnetto S, Boissier S, Delmas PD, Clezardin P. Additive antitumor activities of taxoids in combination with the bisphosphonate ibandronate against invasion and adhesion of human breast carcinoma cells to bone. Int J Cancer 1999;83:263–9.
- Karabulut B, Erten C, Gul MK, Cengiz E, Karaca B, Kucukzeybek Y, . Docetaxel/zoledronic acid combination triggers apoptosis synergistically through downregulating antiapoptotic Bcl-2 protein level in hormone-refractory prostate cancer cells. Cell Biol Int 2009;33:239–46.
- Goffinet M, Thoulouzan M, Pradines A, Lajoie-Mazenc I, Weinbaum C, Faye JC, . Zoledronic acid treatment impairs protein geranyl-geranylation for biological effects in prostatic cells. BMC Cancer 2006;6:60.
- Senaratne SG, Mansi JL, Colston KW. The bisphosphonate zoledronic acid impairs Ras membrane [correction of impairs membrane] localisation and induces cytochrome c release in breast cancer cells. Br J Cancer 2002;86:1479–86.
- Nogawa M, Yuasa T, Kimura S, Kuroda J, Segawa H, Sato K, . Zoledronic acid mediates Ras-independent growth inhibition of prostate cancer cells. Oncol Res 2005; 15:1–9.
- Marra M, Santini D, Meo G, Vincenzi B, Zappavigna S, Baldi A, . Cyr61 downmodulation potentiates the anticancer effects of zoledronic acid in androgen-independent prostate cancer cells. Int J Cancer 2009;125:2004–13.
- Benford HL, Frith JC, Auriola S, Monkkonen J, Rogers MJ. Farnesol and geranylgeraniol prevent activation of caspases by aminobisphosphonates: Biochemical evidence for two distinct pharmacological classes of bisphosphonate drugs. Mol Pharmacol 1999;56:131–40.
- Morgan C,Wagstaff J. Is there a role for ibandronate in the treatment of prostate cancer patients with bony metastases? Acta Oncol 2009;48:882–9.
- Ohlmann CH, Jung C, Jaques G. Is growth inhibition and induction of apoptosis in lung cancer cell lines by fenretinide [N-(4-hydroxyphenyl)retinamide] sufficient for cancer therapy? Int J Cancer 2002;100:520–6.
- Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: A rational approach to clinical protocol design. J Natl Cancer Inst 1994;86:1517–24.
- Caraglia M, Marra M, Naviglio S, Botti G, Addeo R, Abbruzzese A. Zoledronic acid: An unending tale for an antiresorptive agent. Expert Opin Pharmacother 2010;11:141–54.
- Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, . Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 2000; 60:2949–54.
- Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, . Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res 1997;57:3890–4.
- Caraglia M, Santini D, Marra M, Vincenzi B, Tonini G, Budillon A. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr Relat Cancer 2006;13:7–26.
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 1998;13:581–9.
- Nogawa M, Yuasa T, Kimura S, Kuroda J, Segawa H, Sato K, . Zoledronic acid mediates Ras-independent growth inhibition of prostate cancer cells. Oncol Res 2005;15:1–9.
- Ullen A, Lennartsson L, Harmenberg U, Hjelm-Eriksson M, Kalkner KM, Lennernas B, . Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol 2005;44:644–50.
- Fabbri F, Brigliadori G, Carloni S, Ulivi P, Vannini I, Tesei A, . Zoledronic acid increases docetaxel cytotoxicity through pMEK and Mcl-1 inhibition in a hormone-sensitive prostate carcinoma cell line. J Transl Med 2008;6:43.
- Adany I, Yazlovitskaya EM, Haug JS, Voziyan PA, Melnykovych G. Differences in sensitivity to farnesol toxicity between neoplastically- and non-neoplastically-derived cells in culture. Cancer Lett 1994;79:175–9.
- Haldar S, Basu A, Croce CM. Bcl2 is the guardian of microtubule integrity. Cancer Res 1997;57:229–33.
- Miquel K, Pradines A, Favre G. Farnesol and geranylgeraniol induce actin cytoskeleton disorganization and apoptosis in A549 lung adenocarcinoma cells. Biochem Biophys Res Commun 1996;225:869–76.
- Rioja A, Pizzey AR, Marson CM, Thomas NS. Preferential induction of apoptosis of leukaemic cells by farnesol. FEBS Lett 2000;467:291–5.
- Maestre N, Bezombes C, Plo I, Levade T, Lavelle F, Laurent G, . Phosphatidylcholine-derived phosphatidic acid and diacylglycerol are involved in the signaling pathways activated by docetaxel. J Exp Ther Oncol 2003;3:36–46.
- Joo JH, Liao G, Collins JB, Grissom SF, Jetten AM. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res 2007;67:7929–36.
- Joo JH, Jetten AM. Molecular mechanisms involved in farnesol-induced apoptosis. Cancer Lett 2010;287:123–135.