Abstract
Aim. Overall survival after cancer is frequently used when assessing the health care service performance as a whole. While used by the public, politicians, and the media, it is often discarded by clinicians and epidemiologists due to the heterogeneous mix of different cancers, risk factors and treatment modalities. We studied the trend in the Nordic 5-year relative survival and excess mortality for all cancers combined to see if the impact of case-mix and variations between countries in diagnostic methods such as breast screening and PSA testing could explain the lower survival in Denmark. Material and methods. From the NORDCAN database 1964–2003, we defined two cohorts of cancer patients, one excluding non-melanoma skin cancer and another also excluding breast and prostate cancer. We estimated age-standardised incidence and mortality rates, 5-year relative survival, and excess mortality rates for varying follow-up periods, and age-specific 5-year relative survival by country, sex and 5-year diagnostic period. Results. Prostate cancer is the main driver of the incidence increase in men, as do breast cancer in women, whereas cancer mortality in all Nordic countries is declining. The 5-year relative survival ratios are increasing in each Nordic population, but less so in Denmark. Country differences in survival stem mainly from follow-up periods immediately after diagnosis. Adjusting for the case-mix of diagnoses diminished differences a little while exclusion of breast and prostate cancer reduced the gap between countries in survival and excess mortality more considerably, yet post-adjustment, Danish patients still fare worse during the first three months after diagnosis. Conclusion. Adjustment for case-mix and exclusion of sites where diagnostic procedures change the pattern of incidence is important when comparing overall cancer survival across countries, but the correction only explains part of the observed differences in survival. Other factors such as stage at presentation, co-morbidity, tobacco and alcohol consumption are likely contributors.
Cancer is a growing problem in the modern world, with increases both in incidence and mortality. Cancer control planning has long been established as a necessity in order to meet the demands of effective cancer control. Cancer control plans since the first WHO manual in 1992 have concerned the problems in both the developing [Citation1,Citation2] and in developed countries [Citation3]. Well-established population-based monitoring systems of cancer incidence and mortality are essential in order to perform situation analysis on cancer and provide a tool for proper follow-up at the population level of actions taken following the establishment of cancer plans. Population-based estimates of incidence, mortality, survival, and to some extent prevalence, are necessary in this respect [Citation4], and all of these measures in combination are needed to provide effective cancer control actions on the heterogeneous diseases combined into the single entity of cancer.
The Nordic countries, with their long tradition of high quality cancer registration based on effective and rather similar health care systems with equal and free access for their citizens, can provide the data needed to establish and monitor cancer control actions. They are also amongst the highest-resourced in the world, with a long tradition for high quality clinical and epidemiological research, as well as national centralised registration of all citizens based on unique ID numbers. It is possible to learn both from survival studies, from randomised clinical series assessing the effectiveness of treatment, and from population-based studies evaluating health care planning and resource allocation. It is thus possible to demonstrate what has been and what may be achieved at the population level when allocating best practice to the different types of cancer.
The EUROCARE studies [Citation5,Citation6] as well as earlier reports on cancer survival in the Nordic countries [Citation7–12] demonstrated survival for specific cancer sites was below that observed in clinical randomised trials. Randomised clinical trials are conducted on carefully selected patients fulfilling preset criteria to minimise differences in the study arms other than the intervention under study, and such patients rarely reflect the general population composition with respect to age, co-morbidities, and other factors influencing survival. Clinicians unfortunately tend to discard the findings from the observational population-based series due to the obvious limitations with crude classifications, lack of detailed stage and treatment information, and suspected lack of comparability across health care systems and national borders. As Berrino et al. [Citation4] state, clinicians often forget the relevance of the population-based survival as a way of benchmarking their own performance, while politicians and administrators under-appreciate the value of population-based estimates when setting provisions for cancer care and deciding resource allocation, given a growing and longer-living cancer patient population, coupled with the emerging need for targeted and expensive therapies.
Both the Nordic and the EUROCARE studies demonstrate variations in country-specific survival for many cancer sites and for all cancers combined. As a general feature, Denmark has a lower survival compared to the other Nordic countries, but at a level comparable to the survival in the UK [Citation5]. This has led to a number of studies attempting to explain the reasons behind the differences. Dickman et al. [Citation13] estimated the potential impact of reducing regional and social class variation; Robinson et al. [Citation14] interpreted the effect of incomplete registration and the effect of cases based on death certificate only. However, neither regional differences nor technical aspects could explain the observed survival differences. Stage of cancer is established as an important predictor of survival, and even if stage is heterogeneously reported and missing for a high proportion of reported cases, adjustment for stage does not appear to fully account for the differences in survival [Citation8]. Morphology, as reported to the cancer registries, has been studied for lung cancer but did not explain the survival discrepancies [Citation10]. However in a breast cancer study, most of the observed survival variations between Denmark and Sweden were explained by adjusting for stage, diagnostic method, and patho-anatomical prognostic variables [Citation11,Citation12].
Epidemiologists and clinicians acknowledge that cancer as a single entity is problematic due to the complex mixture of different cancer types and subtypes with varying underlying risk factors, diagnostic methods and therapies. For these reasons the measures of overall incidence, mortality and survival attract limited professional interest. However, it must be realised that the public, journalists, politicians, and administrators often see this differently and take a general view of cancer when assessing the cancer burden and impact of cancer control plans, and use summary measures such as overall survival. Cancer plans are now developed or being developed in many countries – including the Nordic populations. When studying cancer within a country or administrative area via internal comparisons, many of the quality issues may be less important. However, when benchmarking is made across administrative borders – between countries, for example – it is very important that data are comparable. The purpose of the NORDCAN database and program is to provide as comparable data as possible from the Nordic countries and to make the results and underlying data available to all potential users [Citation15]. Neither epidemiologists nor clinicians place a major emphasis on the group “all cancers” and hence only brief reports on this entity appear [Citation5]; however, we analysed the overall cancer survival in the Nordic countries to evaluate the validity of this measure. An emphasis was placed on the case-mix of diagnoses (i.e. the variation between countries in the distribution of incidence of cancers that differ in terms of lethality) and the impact of screening for breast cancer and increased diagnostics resulting from use of the prostate specific antigen (PSA) test for prostate cancer, in order to visualise the collective impact on the interpretation of the trends in overall survival after a cancer diagnosis.
Material and methods
We used the NORDCAN database, and hence the data have been checked and converted to well-defined entities, as described by Engholm et al. [Citation15,Citation16]. We included all cancers for patients diagnosed 1964–2003 with malignant neoplasms (ICD 10: C00-C95+D09.0+D41.4+D32-33+D42-43) excluding non-melanoma skin cancer (ICD10: C44+C46.0) in Denmark, Finland, Iceland, Norway, and Sweden, and supplemented the cancer records with individual records for death and emigration up to the end of 2006.
The data were analysed in two ways, first for all cancer sites excluding non-melanoma skin cancer and second for all cancer sites excluding non-melanoma skin cancer, prostate and breast cancer. The case-mix was considered since the frequency distribution of cancer sites deviate between countries; for instance, rectal cancer has been less frequent in Iceland and more frequent in Norway. Prostate cancer incidence has changed radically in some countries in the last two decades due to an increased use of PSA tests and subsequent biopsies, but less so in Denmark than in the other Nordic countries [Citation17]. Breast cancer incidence is influenced by screening with a peak in incidence due to earlier diagnosis following the start of screening in the screened age groups. The start of breast cancer screening varied between countries, and by the end of the study period in 2003, it was still only offered in four of 16 counties in Denmark. With this in mind, a common weighting of cancer sites, a case-mix adjustment, should be used when comparing survival for summary groups between countries. We used as weights the Nordic distribution of the 15 most common cancer sites and the residual group of cancers in the last 5-year period 1999–2003 for each sex. Two sex-specific weight distributions for the two different summary groups all cancer sites but non-melanoma skin and all cancer sites but non-melanoma skin, prostate, and breast cancer as can be seen in .
Table I. Weights (%) used in case-mix adjustment of cancer sites for 5-year relative survival for the summary groups All cancer sites but non-melanoma skin (W 1) and All cancer sites but non-melanoma skin, prostate and breas t cancer(W2) by sex. Nordic cancer survival study 1964–2003.
As described in detail elsewhere [Citation18], we used the cohort survival method for the first seven 5-year periods in each country from 1964–1998, and a hybrid analysis combining period and cohort survival in the last period 1999–2003 [Citation19]. Country-specific life tables were used to calculate the expected survival. Age standardisation was performed using the standard weight distribution for cancers more common among the elderly (ICSS standard number 3) as in the EUROCARE-4 analysis [Citation20]. Patients were followed until death, emigration or loss to follow-up, or to the end of 2006. Excess mortality rates were stratified into short intervals after diagnosis: the first month, 1–3 months, 4–12 months and yearly intervals thereafter. We present age-standardised (World) incidence and mortality rates, age- standardised (ICSS) 5-year relative survival ratios and excess mortality rates for the follow-up periods, the first month, 1–3 months, and 2–5 years following diagnosis, as well as age-specific 5-year relative survival ratios by country, sex, and age taking case-mix into account. The analysis of all sites combined without adjustment for case-mix is presented by Engholm et al. [Citation18].
Results
Excluding non-melanoma skin cancer, a total of 1 678 631 cancers among men and 1 681 766 among women were included in the study of all cancers. Excluding prostate and breast cancer, the total number of cases among men was reduced by 21.6% to 1 316 724 and among women by 25.9% to 1 246 873. presents the incidence, mortality and survival for all cancer sites but non-melanoma skin for men and women, and the equivalent but also excluding breast and prostate cancer. Over the entire study period, incidence was increasing, however for men it is evident on comparing and that much of the increase since the mid-1980s was due to increasing incidence of prostate cancer. Denmark has the highest incidence in 1994–2003 when prostate cancer is excluded, whereas both Norway and Iceland have a higher total cancer incidence than Denmark when prostate cancer is included. It is noteworthy that the incidence of all cancer is declining from 1974 onwards in Finland when prostate cancer is excluded, as is the case for Sweden since the mid-1980s. The exclusion of the mortality of prostate cancer had no impact on the observed cancer mortality pattern between countries. Similarly it is clear that breast cancer was the driving force behind the increasing female incidence rates, however even on excluding breast cancer, incidence still rose, with the highest incidence in Denmark and Iceland, and the lowest rates in Finland. The cancer mortality among women is lower when breast cancer is excluded, with no change in the order of magnitude of the rates between countries.
Figure 1. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for all cancer sites but non-melanoma skin (case-mix adjusted) by sex and country. Nordic cancer survival study 1964–2003.
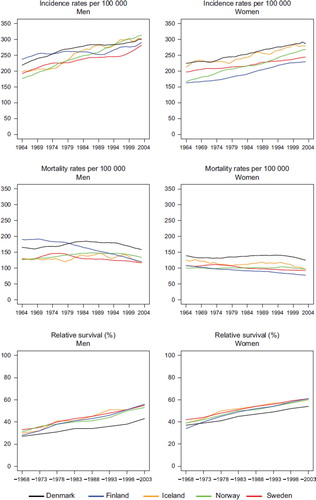
Figure 2. Trends in age-standardised (World) incidence and mortality rates per 100 000 and age-standardised (ICSS) 5-year relative survival for all cancer sites but non-melanoma skin, prostate, and breast cancer (case-mix adjusted) by sex and country. Nordic cancer survival study 1964–2003.
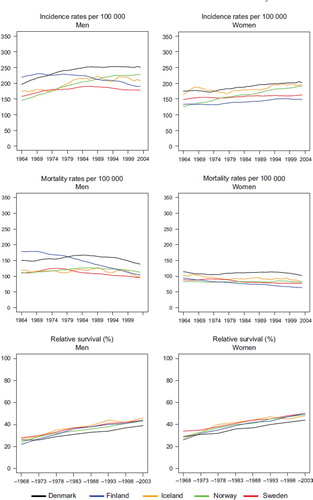
The 5-year relative survival ratio is increasing during the observation period for all countries. Trends however, are quite different in men when prostate is excluded and in women when breast cancer is excluded, although to a lesser extent. The lower 5-year relative survival in Denmark among men is dramatic and difference increasing over time compared to the other Nordic countries. When prostate cancer is excluded the lower Danish survival is observed from the early-1980s, but the absolute differences in survival are not increasing markedly over time. For women the survival levels are lowered almost equally in all Nordic countries when breast cancer is excluded, however, the gap between Denmark and the other countries remains and appears to be increasing.
and give the actual numbers, and show that the 8 to 15 percentage point difference between countries, with lower survival among men in Denmark compared to the other Nordic populations in 1989–1993 has become greater in 1999–2003 compared to Finland and Norway, at the same level compared with Sweden, and reduced slightly relative to Iceland. After excluding prostate cancer, the difference in relative survival was 4–10 percentage points in 1989–1993, and 4–7 percentage for the most recent period 1999–2003. For women, the 3–8 percentage point difference is relatively stable over time, irrespective of whether breast cancer is included or not.
Table II. Trends in survival for all cancer sites but non-melanoma skin (case-mix adjusted) by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
Table III. Trends in survival for all cancer sites but non-melanoma skin, prostate, and breast cancer (case-mix adjusted) by sex and country. Number of tumours (N) included and the 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)). Nordic cancer survival study 1964–2003.
and present the 5-year relative survival ratios by age groups. The 5-year relative survival is highest among the younger male patients (<60 years) and ranges from 61–65% during 1999–2003 in all countries but Denmark, where it reaches 49%. The differences in survival are only marginally lower among the older patients, with a survival of 32% among 80–89 year old male patients in Denmark compared to between 39% and 46% in the other countries, also in the last period. Excluding prostate cancer, the levels of 5-year survival for male patients diagnosed at ages under 50 1999–2003 range from 58 to 65%, with Denmark at 54%, and for ages 80–89, between 30% and 35%, with Denmark at 26%. Comparing the two tables, it is evident that the exclusion of prostate cancer reduces the survival in all countries but less so in Denmark, with the exception of Danish and Icelandic men diagnosed at ages 0–49. For women, the survival pattern is similar to that among men, with the highest survival observed among the younger patients; with the exclusion of breast cancer, survival reduced 6–13 percentage points among women diagnosed below the age of 80 in all countries.
Table IV. Trends in 5-year age-specific relative survival in percent after cancer of all sites but non-melanoma skin (case-mix adjusted) by sex and country. Nordic cancer survival study 1964–2003.
Table V. Trends in 5-year age-specific relative survival in percent after cancer of all sites but non-melanoma skin, prostate, and breast cancer (case-mix adjusted) by sex and country. Nordic cancer survival study 1964–2003.
The excess mortality rates in the first month following diagnosis in and show that the levels are high in the early years around 1970 for the summary group all cancer sites but non-melanoma skin and the levels decrease to about the half in the last period 1999–2003. The differences between countries peak in the 1970s, with Denmark and Sweden having the highest rates. Excess rates then decrease to about the same level in the last period, but with rates in Iceland and Sweden somewhat lower. The pattern is very similar for male and female patients, irrespective of whether prostate and breast cancers are excluded, – although the level for men change. In the second follow-up interval, the second and third month following diagnosis, a decrease in the excess rates is seen over time, but with a considerably lower level and trend than in the first interval. When studying all calendar years, the excess mortality is higher in Denmark than in the other countries. In the last interval shown in , the interval 2–5 years after diagnosis, the levels of excess mortality conditional on the cancer patients surviving the first two years after diagnosis, are much lower, about 6–12 per 100 person-years, with little difference between the countries and a minor decreasing trend over time.
Figure 3. Trends in age-standardised (ICSS) excess death rates per 100 person years for all cancer sites but non-melanoma skin (case-mix adjusted) by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003.
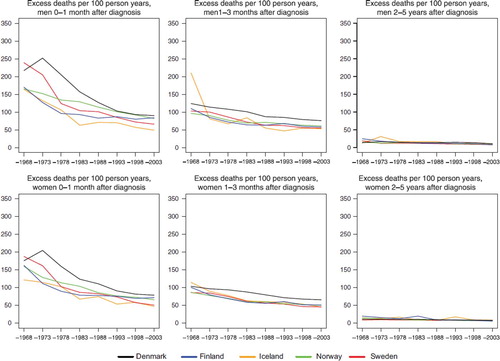
Figure 4. Trends in age-standardised (ICSS) excess death rates per 100 person years for all cancer sites but non-melanoma skin, prostate, and breast cancer (case-mix adjusted) by sex, country, and time since diagnosis in Nordic cancer survival study 1964–2003.
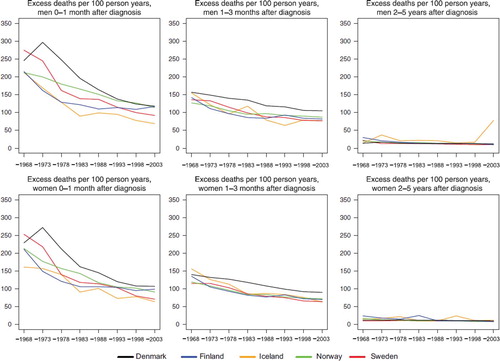
Adjusting for case-mix in the last period 1999–2003, the relative 5-year survival increase for all sites for Danish men (1 percentage points), had no impact on Finnish men, and decreased for Icelandic (2 percentage point), Norwegian (1 percentage point), and Swedish men (4 percent points) (). In women, the adjustment increased the survival in Denmark (2 percentage points), Iceland (3 percentage points), Norway (2 percentage points), and had no impact on the survival in Finland and Sweden. After removing prostate cancer from the all cancer group, adjustment for case-mix had no impact on the Danish survival but increased survival by 4 percent points in Finland and 1 percent point in Iceland, and lowered survival in both Norway and Sweden. In women, the survival increased with case-mix in Denmark and Iceland when breast cancer was removed from the all-cancers figure, and decreased in Finland, Sweden, and Norway.
Table VI. Number of tumours (N) and 5-year age-standardised (ICSS) relative survival in percent with 95% confidence intervals (RS (CI)) with and without case-mix for the summary group all cancer sites but non-melanoma skin and for the summary group when also excluding prostate cancer among men and breast cancer among women. Patients diagnosed 1999–2003 in the Nordic countries by sex.
Discussion
We have used the routinely-collected incidence and mortality data delivered by the Nordic cancer registries to the NORDCAN database [Citation16] to conduct a comprehensive situation analysis on cancer in the Nordic countries, an action recommended by the WHO for cancer control monitoring [Citation3]. Although such summarised data may be of lesser relevance to clinicians from a public health perspective, it is considered of value as a benchmarking exercise examining the performance of the health care system as a whole [Citation4]. However, as quality and comparability of data are of concern, our results should interest all stakeholders in cancer control, both in the Nordic countries and elsewhere, inasmuch that the Nordic cancer registries are recognised for their high quality data, with national central population registries and a unique ID given to every citizen, and where reliable follow-up for vital status can be made using register linkages, thus avoiding biases seen elsewhere from a failure to adequate follow-up or link the relevant data.
In general, we found similar increases in the 5-year relative survival ratios in the Nordic countries from 1964–2003. The gap in survival between Denmark and the other Nordic countries demonstrated from the late 1970s may be considered to result from the growth of financial resources to health care slowing down in Denmark and a new agreement between the Medical Association and the hospital owners that introduced significant changes in working hours for doctors. This gap is still seen in patients diagnosed in 1999–2003, and to some extent the survival disparities have widened over the years, despite the changes introduced in health care in Denmark in relation to the development of the national cancer control plan (launched in 2000). One may argue that trends in and comparison of overall survival have little relevance since it is for an aggregated group of very different diseases, but the measure is of political interest and has been accepted as an indicator for the overall performance of health care systems [Citation4,Citation6]. It is worthy of note, that the survival differences diminish when case-mix is taken into account, particularly for men when prostate cancer is excluded. This is not unexpected and has been discussed by Berrino et al. in a commentary on earlier EUROCARE results [Citation4]. On the other hand, these findings raise a general warning as to the relevance of presenting and comparing overall survival ratios including all cancers, inasmuch that the results are heavily dependent on an in-depth knowledge of diagnostic methods, criteria and medical examination practices, as well as the potential for effective treatment regimens. For prostate cancer, the impact on variation in the use of PSA testing on incidence and survival are well-established [Citation17], but differences in diagnostic intensity are likely for other primary sites as well – e.g. for colorectal cancers and the use of haemocult or endoscopic examinations as screening methods for early cancers [Citation21,Citation22]. For other sites such as cancers of the brain, it has been previously demonstrated [Citation23] that the use of modern imaging techniques including CT and MR scans were introduced first for younger age groups and only after a period of time routinely offered to patients diagnosed at older ages.
It was expected, perhaps naively, that the Danish survival would have improved and converged towards the survival of the other Nordic countries following the initiation of the first Danish cancer plan in 2000 [Citation24]. It could be argued that it is too early to pick up more recent changes, since only the latest three years of incidence data could have been influenced by improvements in treatment, with follow-up time equally short. However, it was clear already in 1998 [Citation8] that a significant part of the survival deficit relates to the first year after diagnosis, and later publications [Citation24–26] showed that this deficit primarily relates to the time window between diagnosis and six months follow-up. Differences in survival tend to diminish where patients have survived the first year [Citation27]. It is thus possible to pick up improvements in diagnostics and treatment early by comparing survival and excess mortality within the first year after diagnosis, whilst waiting for the long-term results of prevention.
Even if survival levels differ between countries, the increase in survival after colorectal cancer in all the Nordic countries, a cancer site where the frontline therapy is surgery, is indicative of modern surgical techniques being implemented at the same pace in all countries. Likewise the similar survival in the Nordic countries observed for lymphomas, sites where chemotherapy remain the first choice, supports the view that chemotherapeutic drugs are provided and administered with rather similar levels of efficiency in all the countries.
In earlier publications, technical reasons for differences in survival have been studied that potentially explained the deficit in Denmark (e.g. regional variations, varying DCO proportions), although such contributions have been considered rather minor [Citation13,Citation14]. The only important factor may have been the lack of linkage in Sweden with the mortality register to follow-back on cases initially only reported on a death certificate [Citation28]. Such a trace-back would likely lower the Swedish survival estimates, but only by a few percent points; even if this were to narrow the gap between Sweden and Denmark, it will not have had any impact on the other countries, where survival remains superior to that of Denmark. Differences in follow-up and missed linkages to mortality data are important issues to consider outside the Nordic area, leading to artificially-inflated survival – but with the use of the unique personal ID in all the Nordic countries, such artefacts are not likely to operate in this study.
The Danish deficit in survival may be considered a true observation, as is supported by the fact that Danish life expectancy has not improved at the same pace observed in the other Nordic countries [Citation18], and is substantially lower at present. The population of Denmark, for example, has a life expectancy more than three years lower than that of Sweden – an observation almost exclusively explained by a higher tobacco and alcohol consumption in Denmark [Citation29]. Since the excess mortality is about the same for the countries after approximately one year, the survival deficit must relate to patient or treatment characteristics close to the time of diagnosis. The survival in Denmark increases almost in parallel with that of the other Nordic countries, the same success – in relative terms – on introducing new therapies and other improvements in management is seen in Denmark as elsewhere. Comparing Denmark, Finland, and Norway – where stage information is included in the cancer registers – the Danes presented with more advanced disease, but had poorer absolute survival levels across stages, and hence adjustment for stage could not explain the overall survival differences [Citation8]. Nevertheless, as stage is one of the most important predictors of survival [Citation8,Citation12,Citation25] the unfavourable Danish stage distribution will be part of the explanation. In order to reduce late stage at presentation, it will be necessary to improve diagnostics considerably, establish organised screening programmes where these are proven cost-effective from a public health perspective, and increase awareness about signs and symptoms, both in the general population and among the medical profession.
A recent analysis of the incidence and survival patterns by socioeconomic position in Denmark showed that tobacco- and alcohol-related cancers were more prominent among the less advantaged socioeconomic groups [Citation30]. Alcohol consumption is lower in Iceland, Norway and Sweden, and with respect to tobacco use, both Sweden and Iceland have low consumption, while smoking trends in Finland have declined rapidly since the 1970s to a level considerably lower than that of Denmark [Citation27]. This may be one explanation for the inferior survival in Denmark, as co-morbidity, related to tobacco and alcohol and a generally poorer state of health will have an impact on the effectiveness of almost all therapeutic modalities. In addition, such risk factors provide the patient with a poorer constitution whereby the tolerance of various therapies is reduced relative to more healthy individuals [Citation31]. Given the obvious societal benefits of reducing tobacco and alcohol consumption in the population, it remains important to aggressively pursue primary prevention policies in each of the Nordic countries, particularly in Denmark.
Acknowledgements
The Nordic Cancer Union (NCU) has financially supported the development of the NORDCAN database and program, as well as the survival analyses in this project.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- WHO. National cancer control programmes; policies and managerial guidelines.Geneva: World Health Organisation; 1992. WHO/CAN/92.1.
- WHO. National cancer control programmes; policies and managerial guidelines.Geneva: World Health Organisation; 1995.
- WHO. National cancer control programmes; policies and managerial guidelines.2nd ed.Geneva: World Health Organisation; 2002.
- Berrino F, Verdecchia A, Lutz JM, Lombardo C, Micheli A, Capocaccia R. Comparative cancer survival information in Europe. Eur J Cancer 2009;45:901–8.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R; the EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Coleman MP, Gatta G, Verdecchia A, Estève J, Sant M, Storm H, . EUROCARE-3 summary: Cancer survival in Europe at the end of the 20th century. Ann Oncol 2003; 14(Suppl 5):v128–49.
- Engeland A, Haldorsen T, Tretli S, Hakulinen T, Hörte LG, Luostarinen T, . Prediction of cancer mortality in the Nordic countries up to the years 2000 and 2010, on the basis of relative survival analysis. A collaborative study of the five Nordic Cancer Registries. APMIS 1995;105(Suppl 49):1–161.
- Engeland A, Haldorsen T, Dickman PW, Hakulinen T, Möller TR, Storm HH, . Relative survival of cancer patients–a comparison between Denmark and the other Nordic countries. Acta Oncol 1998;37:49–59.
- Rørth M, Storm H. [Treatment of cancer patients in the Nordic countries]. Nord Med 1998;13:293–6.
- Storm HH, Dickman PW, Engeland A, Haldorsen T, Hakulinen T. Do morphology and stage explain the inferior lung cancer survival in Denmark? Eur Respir J 1999;13:430–5.
- Christensen LH, Engholm G, Ceberg J, Hein S, Perfekt R, Tange UB, . Can the survival difference between breast cancer patients in Denmark and Sweden 1989 and 1994 be explained by patho-anatomical variables?–a population-based study. Eur J Cancer 2004;40:1233–43.
- Christensen LH, Engholm G, Cortes R, Ceberg J, Tange U, Andersson M, . Reduced mortality for women with mammography-detected breast cancer in east Denmark and south Sweden. Eur J Cancer 2006;42:2773–80.
- Dickman PW, Gibberd RW, Hakulinen T. Estimating potential savings in cancer deaths by eliminating regional and social class variation in cancer survival in the Nordic countries. J Epidemiol Community Health 1997;51:289–98.
- Robinson D, Sankila R, Hakulinen T, Møller H. Interpreting international comparisons of cancer survival: The effects of incomplete registration and the presence of death certificate only cases on survival estimates. Eur J Cancer 2007; 43:909–13.
- Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff ML, Klint Å, . NORDCAN: A Nordic tool for cancer information, planning, quality control, and research. Acta Oncol 2010.
- Engholm G, Ferlay J, Christensen N, Bray F, Klint Å, Ólafsdóttir E, . NORDCAN: Cancer incidence, mortality and prevalence in the Nordic countries, Version 3.3. Association of Nordic Cancer Registries. Danish Cancer Society. 2008. Available from: http://www.ancr.nu
- Kvåle R, Auvinen A, Adami HO, Klint A, Hernes E, Møller B, . Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst 2007;99:1881–7.
- Engholm G, Gislum M, Bray F, Hakulinen T. Trends in the survival of patients diagnosed with cancer in the Nordic countries 1964–2003 followed up the end of 2006. Material and methods. Acta Oncol 2010.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standara dising survival ratios. Eur J Cancer 2004;40:2307–16.
- Hakama M, Hoff G, Kronborg O, Påhlman L. Screening for colorectal cancer. Acta Oncol 2005;44:425–39.
- Hoff G, Grotmol T, Skovlund E, Bretthauer M; Norwegian Colorectal Cancer Prevention Study Group. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: Randomised controlled trial. BMJ 2009;338:b1846.
- Muir CS, Storm HH, Polednak A. Brain and other nervous system tumours. Cancer Surv 1994;19–20:369–92.
- Storm HH, Gislum M, Engholm G. [Cancer survival before and after initiating the Danish Cancer Control plan]. Ugeskr Laeger 2008;170:3065–9.
- Folkesson J, Engholm G, Ehrnrooth E, Kejs AM, Påhlman L, Harling H, . Rectal cancer survival in the Nordic countries and Scotland. Int J Cancer 2009;125:2406–12.
- Engholm G, Kejs AM, Brewster DH, Gaard M, Holmberg L, Hartley R, . Colorectal cancer survival in the Nordic countries and the United Kingdom: Excess mortality risk analysis of 5 year relative period survival in the period 1999 to 2000. Int J Cancer 2007;121:1115–22.
- Storm HH, Engholm G, Hakulinen T, Tryggvadóttir L, Klint Å, Gislum M, . Survival of patients diagnosed with cancer in the Nordic countries up to 1999–2003 followed to the end of 2006. A critical overview of the results. Acta Oncol 2010.
- Mattsson B, Wallgren A. Completeness of the Swedish Cancer Register. Non-notified cancer cases recorded on death certificates in 1978. Acta Radiol Oncol 1984;23:305–13.
- Juel K. [Life expectancy and mortality in Denmark compared to Sweden. What is the effect of smoking and alcohol?] Ugeskr Laeger 2008;170:2423–7.
- Dalton SO, Schüz J, Engholm G, Johansen C, Kjaer SK, Steding-Jessen M, . Social inequality in incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: Summary of findings. Eur J Cancer 2008;44:2074–85.
- Tønnesen H, Nielsen PR, Lauritzen JB, Møller AM. Smoking and alcohol intervention before surgery: Evidence for best practice. Br J Anaesth 2009;102:297–306.