Abstract
Background. Complementary and alternative medicine (CAM) use is common among breast cancer patients. Several CAM therapies may have negative side effects or interact with conventional therapies. We studied biologically based CAM use with and without vitamins/minerals in relation to patient and tumor characteristics as well as treatment in an ongoing prospective cohort of 855 primary breast cancer patients. Methods. Patients from two hospitals in southern Sweden were included. Pre-operative and follow-up questionnaires containing questions on food intake, lifestyle, and concomitant medications, including natural remedies, were completed up to five years postoperatively. Clinical information was obtained from clinical records and tumor characteristics from pathology reports. Results. CAM and/or vitamins/minerals were used by 34.2% pre-operatively and by 57.9% during at least one visit. Over 100 different preparations were reported. At least eight of the commonly used preparations may interact with conventional breast cancer therapies. CAM users more often had a BMI <25 kg/m2 (OR 1.76; 95%CI 1.33–2.33), were more often nulliparous (OR 1.59; 1.08–2.34), alcohol (OR 2.13; 1.44–3.14), antidepressants (OR 1.48; 1.02–2.15), and hormone therapy users (OR 1.57; 1.18–2.07), less often smokers (OR 0.71; 0.50–0.99), and consumed less coffee (OR 0.88; 0.82–0.95) than non CAM users. Tumor characteristics were not associated with CAM use. CAM use was more common among tamoxifen (OR 1.32; 1.00–1.75) and less common among chemotherapy (OR 0.63; 0.42–0.92) treated patients. Vitamins/minerals use was more common in aromatase inhibitor treated patients (OR 1.84; 1.33–2.53). There was no significant association between short-term disease-free survival and CAM use. Conclusion. CAM use was common and associated with certain patient characteristics. CAM use may cause clinically significant drug interactions and it is therefore of clinical interest to identify potential CAM users.
A large number of breast cancer patients use complementary and alternative medicine (CAM), often unbeknownst to their physicians [Citation1–3]. There are few well-designed clinical trials and observation studies to determine the effects of CAM and antioxidants among cancer patients [Citation4–6]. CAM users often report concomitant use of more than one CAM, which makes it difficult to evaluate the clinical effects of specific CAMs [Citation7–9]. According to the National Center for Complementary and Alternative Medicine (NCCAM), CAM is a wide group of different medical and health care systems, practices and products that are not considered as conventional medicine [Citation10]. The prevalence of CAM use among newly diagnosed breast cancer patients in the USA has been reported to be up to 86% [Citation9]. Another study reported that 28.1% of newly diagnosed patients with an early-stage breast cancer started using CAM after diagnosis [Citation7]. A recent Danish study reported that 40% of newly diagnosed breast cancer patients used CAM and the most common CAMs were dietary- or vitamin supplements (27.5%) and herbal medicine (9.6%) [Citation11]. A large Swedish study reported that almost 40% of 2 974 women from the general population used CAM including vitamins and minerals and that CAM use is becoming more and more common [Citation12]. A pilot study based on a subset of 233 patients of the present study population was conducted by Malekzadeh et al. 2005 [Citation13] and showed that CAM use was also common in Sweden, but the follow-up time was only one year and no survival analysis was performed.
Several studies have described the typical CAM users as younger, better educated, of higher socioeconomic status and more health conscious than non-users [Citation7–9,Citation11]. Some studies have associated CAM use with higher psychosocial distress, poorer quality of life and depression, as reviewed by DiGianni et al. [Citation14] as well as greater fear of recurrence and a higher perceived risk of metastasis and death [Citation15]. A large prospective cohort of 1 000 breast cancer patients did not find an association between CAM use prior to diagnosis, and tumor characteristics or treatment [Citation9].
Many CAM therapies, especially biologically based CAMs, have been reported to have negative side effects, due to intrinsic toxicity and drug interactions, and may diminish the therapeutic response to conventional therapy and even promote resistance to anti-cancer drugs [Citation2,Citation3,Citation5,Citation16].
We aimed to study the general use of biologically based CAMs among breast cancer patients. More specifically, we aimed to study the characteristics of CAM users and compare use of concomitant medications such as antidepressants, tumor characteristics and breast cancer treatment between CAM users and non-users.
Materials and methods
Study design and materials
Women assessed pre-operatively at the Lund University Hospital and the Helsingborg Hospital, Sweden, for a first breast cancer were invited to take part in an ongoing study regarding genetic and non-genetic factors that could be associated with breast cancer prognosis and treatment response. Patients were included between October 2002 and December 2008 in Lund and between April 2006 and December 2008 in Helsingborg. Women were invited to participate regardless of ethnic background, age and stage. The vast majority of women included were ethnic Swedes. Patients who had had breast cancer earlier or who had been diagnosed and treated for another type of cancer within the past 10 years were not eligible to participate. The Ethics Committee of Lund University approved the study. Written informed consents were obtained from all patients.
Data collection
Body weight, height, waist and hip circumferences and breast volumes were measured at the pre-operative visit. For the majority of the patients, the preoperative visit took place less than a week prior to surgery. All patients filled out a pre-operative questionnaire including questions on birth date, coffee consumption, smoking, alcohol intake, reproductive history and family history of cancer, use of exogenous hormones and concomitant medications during the past week. The patients have clinical follow-up visits up to three years postoperatively. Follow-up questionnaires were completed at three to six months, and one, two, three and five years postoperatively.
Assessment of CAM use
The question regarding use of concomitant medications was open and the patients were asked to write down all the medications they had consumed during the past week, including natural remedies and dietary supplements. Some women wrote for example “phytoestrogens”, “herbal remedy against hairloss”, “herbal remedy against radiation therapy side-effects” etc. For some of the preparations, we were unable to identify the ingredients, but all women reporting any kind of CAMs were classified as CAM users. The patients were also asked about their food intake the same day.
We included biologically based CAM in this study, which according to NCCAM includes botanicals, animal-derived extracts, vitamins, minerals, fatty acids, amino acids, proteins, prebiotics and probiotics, whole diets, and functional foods [Citation10]. We did not specifically enquire about special diets, instead the participants filled in the questionnaire what they had eaten the same day. Many of the patients wrote down biologically based CAMs they had used under the question inquiring about their food intake the same day, such as flaxseed oil or different soy products. We also included the use of Mistletoe injections. Biologically based CAMs are referred to as CAM throughout the paper. Many vitamin and mineral preparations contained both vitamins and minerals, and these two were therefore combined into one group, vitamins/minerals.
Other covariates
Information including type of surgery, adjuvant treatment, sentinel node biopsy and axillary node dissection was obtained from each patient's chart. Pathological tumor size (pT), histological type and grade, axillary node involvement (pN), signs of distant metastases, estrogen receptor (ER) and progesterone receptor (PR) status were obtained from each patient's pathology report. Information on breast cancer events, defined as local or regional recurrence, new breast cancer, or distant metastasis was obtained from each patient's chart and pathology report. All tumors were analyzed at the Department of Pathology of Lund University Hospital or Helsingborg Hospital. HER-2/neu status was routinely analyzed as of November 2005.
Study population
There are nine hospitals in the South Swedish Health Care Region performing breast cancer surgery. The Lund University Hospital catchment area serves almost 300 000 inhabitants and the Helsingborg Hospital, located approximately 50 km north of Lund, serves another 250 000 inhabitants. Breast cancer patients are not referred to other hospitals for surgery. According to data obtained from the Regional Tumor Registry (between October 1, 2002 and December 31, 2007) and from The Information Network for Cancer Care (INCA) (between January 1, 2008 and December 31, 2008), 1 132 women with breast cancer were registered in Lund and received surgery, of which 654 (57.8%) were included in our study. The mean age for patients who received surgery in Lund was 60.5 years. Among all patients who received surgery in Lund and where hormone receptor status was available, 900 (84.9%) had estrogen receptor (ER) positive tumors, 160 (15.1%) had ER negative tumors, 718 (68.5%) had progesterone receptor (PR) positive tumors and 330 (31.5%) had PR negative tumors. Between April 1, 2006 and December 31, 2008, 568 women with breast cancer were registered in Helsingborg and received surgery of which 201 (35.4%) were included in our study. The mean age for patients who received surgery in Helsingborg was 62.5 years. Among all patients who received surgery in Helsingborg and where hormone receptor status was available, 454 (84.4%) had ER positive tumors, 84 (15.6%) had ER negative tumors, 331 (61.6%) had PR positive tumors, and 206 (38.4%) had PR negative tumors.
Of the patients that had not suffered breast cancer events and were scheduled for follow-up visits by December 31, 2008, 95% were included at the three to six months follow-up, 92% were included at the one year follow-up, 87% were included at the two year follow-up, 83% were included at the three year follow-up and 77% were included at the five year follow-up visit. Fifteen patients were registered as dead from non-breast cancer related causes.
Data analyses
The statistical software PASWStatistics/SPSS 17.0 was used for all statistical analyses. Vitamins and minerals were analyzed as one variable, vitamins/minerals. Logistic regression models were used to calculate odds of CAM use in relation to patient and tumor characteristics. The logistic regression models to calculate the odds of CAM use in relation to tumor characteristics were adjusted for tumor size, ER status, PR status, nodal involvement, nulliparity, BMI and age. Interaction variables were calculated between the hospital and patient characteristics in order to detect interactions between the two hospitals in relation to CAM use and patient characteristics. Kaplan-Meier and Cox regression models were used to compare breast cancer-free survival in relation to CAM use, adjusted for age, tumor size, nodal involvement, and histological grade. Breast cancer-free survival was calculated from 0.3 years to the last study follow-up or to a diagnosis of a breast cancer event prior to December 31, 2008. Since some women had received preoperative treatment in the form of neoadjuvant therapy or interstitial laser thermotherapy we added dummy categories to the pT (neo and laser) and pN (neo) in order to be able to include these patients in the Cox regression models. Since there were few women with large or advanced tumors, pT3 or pT4, we combined these into pT2+. We analyzed disease-free survival in patients with invasive tumors in relation to any CAM and/or vitamins/minerals use reported at the preoperative and/or at the three to six months follow-up visit using Kaplan-Meier and Cox regression models. We also analyzed disease-free survival in relation to reported concomitant use of any CAM and/or vitamins/minerals concomitant with radiation therapy or chemotherapy at the three to six months follow-up visit. Similarly, we analyzed disease-free survival in patients with ER positive invasive tumors with and without reported use of CAM and/or vitamins/minerals concomitant with tamoxifen treatment at the three to six months follow-up visit and at the one year follow-up visit. A p-value of <0.05 was taken to be significant. All p-values were two-sided.
Results
Information on CAM use was available for 846 of the 855 patients. For nine patients, we could not read the handwriting or identify the reported medications. Pre-operatively, 21.3% of patients used a biologically based CAM, excluding vitamins/minerals supplements. Overall, 38.7% of the women reported the use of CAM excluding vitamins/minerals supplements during at least one visit. In the 269 patients who ever used CAM excluding vitamins/minerals supplements and who had been followed up to the one year visit, 126 patients (47%) had used CAM during 50% or more of their visits and 40 patients (15%) reported CAM use during all their visits. CAM and/or vitamins/minerals supplements were used by 34.2% pre-operatively and by 57.9% of the patients during at least one visit. The patients reported over 100 different preparations, and the most common preparations are shown in . At least eight of the commonly used preparations (echinacea, flaxseed, omega-3, garlic, ginkgo, ginseng, green tea, and soy products) may interact with conventional breast cancer therapies either through CYP enzymes, drug transporters or through stimulation of the estrogen receptor (ER) or regulation of the HER2/neu oncogene expression. Other CAMs that may cause drug interactions and affect tumor biology, such as phytoestrogens, St John's Wort and omega-6, were only reported by a few patients.
Table I. The most common CAMs reported by different patients at baseline, three to six months, one, two, three and five years after the primary surgery. Overall, over 100 different preparations were reported by the women and this table shows only those that were reported at least 10 times.
The characteristics of the 855 CAM users and non-users from Lund and Helsingborg are shown in . CAM use did not differ according to age. CAM users were more likely to have a normal BMI under 25 kg/m2, OR 1.76 (95%CI 1.33–2.33; p<0.0001) while smoking was less common OR 0.71 (95%CI 0.50–0.99; p=0.045). CAM users also consumed less coffee OR 0.88 (95% CI 0.82–0.95; p=0.002) per cup per day. CAM users were more often nulliparous OR 1.59 (95%CI 1.08–2.34; p=0.019) and hormone replacement therapy (HRT) was more common among CAM users than non-users, OR 1.57 (95%CI 1.18–2.07; p=0.002). Moreover, alcohol and antidepressant use was more common among CAM users, OR 2.13 (95%CI 1.44–3.14; p=0.0001) and OR 1.48 (95%CI 1.02–2.15; p=0.040), respectively. Neither anxiolytic nor sleeping pill use was significantly associated with CAM use.
Table II. Patient characteristics in relation to CAM use at any visit, OR (95%CI) for being a CAM user during at least one visit. Patient characteristics as reported at the first visit, unless otherwise indicated.
The tumor characteristics of 812 patients who neither received neoadjuvant therapy nor interstitial laser thermotherapy are shown in . Tumor size and hormone receptor status were not associated with CAM use. It was less common that CAM users had histological grade three, OR 0.65 (95%CI 0.46–0.90; p=0.010). However, this was no longer significant after adjustment for tumor size, ER status, PR status, nodal involvement, nulliparity, BMI and age.
Table III. Tumor characteristics of the 812 women included in the study who did not receive neoadjuvant therapy (n=31) or interstitial laser thermotherapy (n=11, information missing from one patient) prior to the operation.
The treatment types for the patients in our cohort are shown in . CAM users were more likely to have received tamoxifen treatment than non-users, OR 1.32 (95%CI 1.00–1.75; p=0.051), although this was only of borderline significance, and less likely to have had postoperative chemotherapy, OR 0.63 (95%CI 0.42–0.92; p=0.019). CAM use was not associated with aromatase inhibitor treatment, neoadjuvant treatment or radiation therapy. Vitamins/minerals supplement users were more likely to have received radiation therapy, OR 1.32 (95%CI 1.01–1.74; p=0.044) and aromatase inhibitors, OR 1.84 (95%CI 1.33–2.53; p=0.0002), respectively. Vitamins/minerals supplement use was not associated with tamoxifen treatment, neoadjuvant treatment or postoperative chemotherapy.
Table IV. Breast cancer treatment among CAM users and non-users in Lund and Helsingborg. Some patients received more than one treatment.
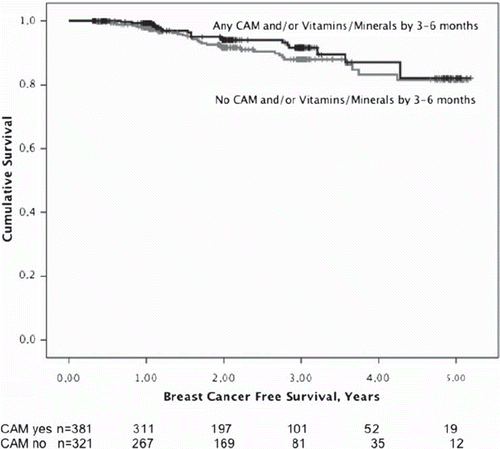
The median follow-up time for all 855 patients was 20 months (interquartile range 8.5–36). We analyzed the breast cancer free survival in relation to CAM use in patients with invasive tumors who had at least 0.3 years of follow-up. We thereby excluded three patients who had signs of distant metastasis on postoperative metastasis screens within three months of surgery. A breast cancer event was defined as local or regional recurrence, new breast cancer, or distant metastasis. We first analyzed disease-free survival in patients with invasive tumors in relation to any CAM and/or vitamins/minerals use reported at the preoperative and/or at the three to six months follow-up visit. Among these 702 patients there were 52 events and there was no significant difference in disease-free survival with respect to early use of CAM and/or vitamins/minerals (Log-rank p=0.32), adjusted Hazard Ratio (HR) 0.78 (95%CI 0.44–1.37; p=0.38), adjusting for age, tumor size, nodal involvement, and histological grade (). We then analyzed disease-free survival in relation to reported concomitant use of any CAM and/or vitamins/minerals with radiation therapy at the three to six months follow-up visit. Among these 329 patients, there were 27 events and there was no significant difference in disease-free survival (Log-rank p=0.69), adjusted HR 1.22 (95%CI 0.56–2.69; p=0.62). Similarly, there was no significant difference in disease-free survival in the 108 patients with postoperative chemotherapy at the three to six months follow-up visit with and without reported concomitant use of CAM and/or vitamins/minerals (Log-rank p=0.10) adjusted HR 1.00 (95% CI 0.28–3.58; p=1.00). Neither was there any significant difference in disease-free survival in the 206 patients with ER positive tumors with and without concomitant reported use of CAM and/or vitamins/minerals and tamoxifen treatment at the three to six months follow-up visit (Log-rank p=0.30) adjusted HR 1.90 (95%CI 0.59–6.09; p=0.28) nor in the 281 tamoxifen-treated patients at the one year follow-up visit (Log-rank p=0.28) adjusted HR 0.61 (95%CI 0.18–2.00; p=0.41).
Discussion
To our knowledge, the present study is the first large prospective study on long-term CAM use among breast cancer patients after diagnosis. The main finding of this prospective cohort study was that CAM use was common among breast cancer patients in Sweden and was associated with certain patient characteristics. Nearly 40% reported use of biologically based CAMs and almost 60% reported use of CAM with and without vitamin/mineral supplements. Pre-operative CAM use, excluding vitamins and minerals, was four times more common among women in this study compared to healthy women from Sweden [Citation12]. In our study, we observed that CAM use remained constant during the follow-up time from the pre-operative visit until five years postoperatively. CAM use may increase after breast cancer diagnosis [Citation7] and is higher among breast cancer patients than among unaffected women [Citation8]. However, most studies of CAM use among breast cancer patients before and after diagnosis are retrospective. Also, there are few prospective studies of how CAM use changes during the period after diagnosis. A large prospective cohort of breast cancer patients showed that the proportion of CAM users was slightly greater in the period of five years prior to diagnosis than in the few months period between diagnosis and study enrolment [Citation9].
CAM users have been described as younger women with higher education, better socioeconomic status and greater health consciousness than non-users [Citation7–9,Citation11]. In a study of unaffected women from breast cancer families, higher education was associated with biologically based CAM use, but no correlation was found for age and CAM use [Citation17]. We did not observe any association between CAM use and age, which is in contrast to the recent report from a large Danish breast cancer cohort, which found that younger age was associated with CAM use [Citation11]. Since we did not have information on education or socioeconomic status, we were unable to study the relationship between CAM use and these factors.
CAM users were more likely to have a normal BMI under 25 kg/m2 than non-users in this cohort. Moreover, a high coffee consumption and smoking were less common among CAM users. Similarly, others have reported that general health behavior, such as exercise, fruit and vegetable intake, reduced red meat, fat and alcohol use, intentional weight loss, and a lower BMI were associated with CAM use in healthy women from breast cancer families [Citation17] and in breast cancer patients [Citation9,Citation11].
We also observed that CAM users were more likely to be nulliparous than the non-users. This is in line with a recent large prospective cohort from Denmark that showed 30% lower pregnancy and live birth rate in CAM users during assisted reproductive treatment (ART) [Citation18]. In our study, CAM users were more likely to have used HRT even though CAM use was not associated with age, which might indicate that CAM users are more prone to seek medical treatment for their menopausal symptoms. In a prospective cohort of over 3 000 healthy women, the use of HRT was associated with CAM use among white women [Citation19]. Kupferer et al. showed that 45% of women who had discontinued the use of conventional HRT used CAM to alleviate menopausal symptoms [Citation20]. Estrogens are contraindicated as HRT for women with breast cancer and many of the CAM preparations, such as black cohosh, pollen extract, soy products and other phytoestrogen containing products, evening primrose and various herbal mixtures are indicated for alleviation of menopausal symptoms by the manufacturers. However, phytoestrogens derived from, for example soya, which are popular for treating menopausal symptoms in tamoxifen treated women, may increase cell proliferation via the ER, promote tumor progression and counteract the effects of tamoxifen [Citation2]. Phytoestrogens may also negate the effect of aromatase inhibitors. We have not specifically analyzed different CAMs in relation to prior HRT use.
Alcohol and antidepressant use was associated with CAM use in this cohort. Previous studies have shown that CAM use is associated with higher psychosocial stress, poorer quality of life and emotional functioning, and depression [Citation7,Citation14]. The positive association between antidepressant or alcohol and CAM use may be explained by the previous findings. In the current study, we did not measure anxiety, depression or quality of life. However, there are no large prospective cohorts that have studied the concomitant use of antidepressants, anxiolytics, sleeping pills and CAMs.
In this cohort, CAM use was more common among tamoxifen treated patients and less common among patients who received postoperative chemotherapy. Since some antidepressants and biologically based CAMs may inhibit or induce the CYP3A4 enzyme and inhibit the CYP2D6 enzyme involved in tamoxifen activation, it is important to acknowledge the association between antidepressant and CAM use as well as CAM use and tamoxifen treatment [Citation16,Citation21,Citation22]. Patients using antidepressants and/or CAMs concomitantly with tamoxifen may have a worse disease-free survival. The CYP2D6 enzyme that is required for formation of the potent tamoxifen metabolite, endoxifen [Citation23,Citation24] is inhibited by many antidepressants [Citation21,Citation22] and possibly also by some CAMs [Citation3,Citation16]. We also observed that vitamins/minerals supplement use was more common among patients who had received aromatase inhibitors. As many postmenopausal women in Sweden use calcium supplements bought over the counter or prescribed by a physician, this association was expected.
No significant association between CAM use and tumor characteristics was observed in this cohort. This is in line with earlier results by Greenlee et al. [Citation9]. They observed no association between CAM use prior to diagnosis, tumor characteristics and stage or breast cancer treatment in a large prospective cohort of 1 000 breast cancer patients [Citation9].
At least eight of the commonly used preparations in our study (echinacea, flaxseed, omega-3, garlic, ginkgo, ginseng, green tea, and soy products) may interact with conventional breast cancer therapies either through CYP enzymes, drug transporters or through stimulation of the ER or regulation of the HER2/neu oncogene expression [Citation2,Citation3,Citation16,Citation25]. There is a lack of well-designed clinical trials and observation studies to determine the effects of CAM among cancer patients [Citation4–6]. CAM users often report concomitant use of more than one CAM type and this makes it difficult to evaluate the clinical effects of specific CAMs [Citation7–9,Citation11]. This was also observed in our study, where CAM users often reported more than one CAM at the same visit and change of CAMs between visits. Women are often recommended by the CAM manufacturers to use a specific CAM only for a limited amount of time. No significant association was found between CAM and/or vitamins/minerals use and early breast cancer events in our cohort. Since the patients switched between different types of CAM and used several types of CAMs concomitantly it was not possible to perform any meaningful analysis of specific types of CAM in relation to disease-free survival. The lack of association between CAM use and disease-free survival may have been diluted by combining all the biologically based CAMs into one group. Moreover, higher education and socioeconomic status have been associated with CAM use [Citation11,Citation17] and with survival benefit, but the disease-free survival was not better in the CAM group in the present study. One hypothesis is that some CAMs may have interacted with the conventional therapies and rendered them less efficient [Citation2,Citation3,Citation5,Citation16].
There are some shortcomings in our study. We lack information regarding which CAMs the patients used between the follow-up visits. We also lack information on CAM use prior to diagnosis. Since many of the patients wrote down biologically based CAMs they had used under the question inquiring about their food intake the same day, such as flaxseed oil or different soy products, we included these products as CAM use. This question was included in the questionnaire to capture whether or not the patient was fasting at the time of blood draw and not for dietary purposes. We are aware that the usual dietary intake of CAMs may therefore be underreported. We also lack information on the socio-demographic factors, such as income, social status and educational level. There are also many strengths in our study. This is a large ongoing prospective study, with regular follow-up visits up to five years postoperatively. Medication use during the past week was self-reported, and the short recall period minimizes the risk for recall bias and may more accurately reflect actual intake compared to data from clinical records or retrospective interviews. Another strength is that information on tumor characteristics and breast cancer treatment was obtained directly from clinical records and pathology reports and was not self-reported by the patients. CAM use in relation to patient and tumor characteristics as well as treatment was analyzed for all women as one group. We recognize that there were some differences between patients from Lund and Helsingborg with respect to use of sleeping pills, anxiolytics and antidepressants, but there were no significant interactions. However, there were fewer patients from Helsingborg than from Lund and the follow-up time in Helsingborg was shorter. The patients included in the cohort were similar to patients not included with respect to ER and PR status, but were somewhat younger than the general breast cancer patient population at these two hospitals. Since age was not associated with CAM use, we do not believe that the age difference would have influenced the results. Moreover, the results remained essentially the same when our models were adjusted for age.
In conclusion, CAM use was common among breast cancer patients in Sweden and was associated with certain patient characteristics, such as alcohol and antidepressant use. Since there are potential drug interactions between conventional medical therapy and certain types of CAMs, it is of clinical importance to identify potential CAM users and discuss potential risks and benefits during ongoing cancer treatments with each patient.
Acknowledgements
This study was supported by grants from The Swedish Cancer Society, The Swedish Research Council, The Medical Faculty at Lund University, The Mrs. Berta Kamprad's Foundation, The Gunnar Nilsson Foundation, The Crafoord Foundation, The South Swedish Health Care Region (Region Skåne ALF), BRO, and Lund Hospital Fund. We thank our research nurses Maj-Britt Hedenblad, Karin Henriksson, Anette Möller, Linda Ågren, Anna Weddig, Ulrika Midelund, Arnhild Nilsson, and Karina Sandström. We thank Dr. Eric T. Dryver for proofreading the manuscript. No conflicts of interest are to be declared.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: A qualitative study in women with breast cancer. J Fam Pract 1999; 48:453–8.
- Gerber B, Scholz C, Reimer T, Briese V, Janni W. Complementary and alternative therapeutic approaches in patients with early breast cancer: A systematic review. Breast Cancer Res Treat 2006;95:199–209.
- Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: Potential adverse interactions with anticancer agents. J Clin Oncol 2004;22:2489–503.
- Jacobson JS, Workman SB, Kronenberg F. Research on complementary/alternative medicine for patients with breast cancer: A review of the biomedical literature. J Clin Oncol 2000;18:668–83.
- Greenlee H, Hershman DL, Jacobson JS. Use of antioxidant supplements during breast cancer treatment: A comprehensive review. Breast Cancer Res Treat 2009;115: 437–52.
- Bardia A, Barton DL, Prokop LJ, Bauer BA, Moynihan TJ. Efficacy of complementary and alternative medicine therapies in relieving cancer pain: A systematic review. J Clin Oncol 2006;24:5457–64.
- Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med 1999;340:1733–9.
- DiGianni LM, Kim HT, Emmons K, Gelman R, Kalkbrenner KJ, Garber JE. Complementary medicine use among women enrolled in a genetic testing program. Cancer Epidemiol Biomarkers Prev 2003;12:321–6.
- Greenlee H, Kwan ML, Ergas IJ, Sherman KJ, Krathwohl SE, Bonnell C, . Complementary and alternative therapy use before and after breast cancer diagnosis: The Pathways Study. Breast Cancer Res Treat 2009;117:653–65.
- NCCAM. National Center for Complementary and Alternative Medicine. 2009 [updated 2009 Jan 16; cited 2009 Jan 16]; Available from: http://nccam.nih.gov/health/whatiscam/overview.htm.
- Pedersen CG, Christensen S, Jensen AB, Zachariae R. Prevalence, socio-demographic and clinical predictors of post-diagnostic utilisation of different types of complementary and alternative medicine (CAM) in a nationwide cohort of Danish women treated for primary breast cancer. Eur J Cancer 2009;45:3172–81.
- Nilsson M, Trehn G, Asplund K. Use of complementary and alternative medicine remedies in Sweden. A population-based longitudinal study within the northern Sweden MONICA Project. Multinational Monitoring of Trends and Determinants of Cardiovascular Disease. J Intern Med 2001;250:225–33.
- Malekzadeh F, Rose C, Ingvar C, Jernström H. [Natural remedies and hormone preparations—potential risk for breast cancer patients. A study surveys the use of agents which possibly counteract with the treatment]. Lakartidningen 2005;102:3226–8, 30–1.
- DiGianni LM, Garber JE, Winer EP. Complementary and alternative medicine use among women with breast cancer. J Clin Oncol 2002;20(Suppl 18): S34–8.
- Rakovitch E, Pignol JP, Chartier C, Ezer M, Verma S, Dranitsaris G, . Complementary and alternative medicine use is associated with an increased perception of breast cancer risk and death. Breast Cancer Res Treat 2005;90: 139–48.
- Nowack R. Review article: Cytochrome P450 enzyme, and transport protein mediated herb-drug interactions in renal transplant patients: Grapefruit juice, St John's Wort - and beyond! Nephrology (Carlton) 2008;13:337–47.
- Myers CD, Jacobsen PB, Huang Y, Frost MH, Patten CA, Cerhan JR, . Familial and perceived risk of breast cancer in relation to use of complementary medicine. Cancer Epidemiol Biomarkers Prev 2008;17:1527–34.
- Boivin J, Schmidt L. Use of complementary and alternative medicines associated with a 30% lower ongoing pregnancy/live birth rate during 12 months of fertility treatment. Hum Reprod 2009;24:1626–31.
- Bair YA, Gold EB, Zhang G, Rasor N, Utts J, Upchurch DM, . Use of complementary and alternative medicine during the menopause transition: Longitudinal results from the Study of Women's Health Across the Nation. Menopause 2008;15:32–43.
- Kupferer EM, Dormire SL, Becker H. Complementary and alternative medicine use for vasomotor symptoms among women who have discontinued hormone therapy. J Obstet Gynecol Neonatal Nurs 2009;38:50–9.
- Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006;25: 1679–91.
- Kirchheiner J, Brosen K, Dahl ML, Gram LF, Kasper S, Roots I, . CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: A first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001; 104:173–92.
- Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: Prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 2004;310:1062–75.
- Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, . Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 2003;95:1758–64.
- Menendez JA, Lupu R, Colomer R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur J Cancer Prev 2005;14:263–70.