Abstract
Objective. The aim is to estimate the future lung cancer incidence in Poland and Finland based on forecasts on hypothetical changes in smoking habits. Material and methods. Data on population, lung cancer and smoking prevalence come from known sources. The simulation model utilized for forecasting was based on smoothing the smoking habit – specific risk ratios estimated for males and females in Europe. Results. Depending on the analyzed scenario in 2030 in Poland mortality rates among men would range from 8 to 125/105 and among women from 7 to 47/105; in Finland among men 5 to 60/105 and among women 4 to 22/105. Conclusions. The results obtained clearly indicate that cutting down on the number of smokers translates directly into a considerable reduction of the lung cancer incidence rate.
Lung cancer is the most common cancer among males worldwide and one of the most common cancers among females. Among men, the highest incidence rates are observed in Europe (especially Eastern Europe) as well as in North America. In female populations, high incidence rates are found in North America and in Europe, particularly Northern and Western Europe.
It is estimated that there were approximately 386 000 incident cases of lung cancer in Europe in the year 2006 – 292 000 among men and 94 000 among women. The number of lung cancer deaths in Europe was about 335 000 (253 000 among men and 82 000 among women) [Citation1].
Among males, both incidence and mortality are highest in Eastern Europe – considerably higher than in other parts of the continent [Citation2]. Among females, the highest incidence and mortality rates are found in Northern Europe (almost twice as high as in Western Europe, and even more than in Eastern and Southern Europe) [Citation3].
Although there are several risk factors influencing risk of developing lung cancer, tobacco smoking is well established as the main cause of this disease [Citation4]. An extensive research programme commenced by Sir Richard Doll back in the 1950s and continued by numerous scientists have led to conclusive evidence that there is a link between smoking tobacco and lung cancer [Citation5]. The studies conducted have also helped to determine the risk of lung cancer incidence depending on the intensity and time period of smoking. At the same time, it has also been possible to demonstrate the beneficial effects of giving up this habit and reducing the risk of contracting lung cancer [Citation6].
There have been significant changes in tobacco consumption and prevalence in Europe during last three decades, especially in Poland [Citation7,Citation8] and Finland [Citation9,Citation10] . These changes have already had and will have in future a major impact on the incidence of lung cancer in the Polish and Finnish populations.
Our paper aims to describe and analyze changes in lung cancer occurrence in Poland and Finland in view of past and future changes in tobacco smoking in the Polish and Finnish populations. Special emphasis will be paid on changes in time trends and role of smoking habits in these changes.
The approach used to predict future changes in lung cancer mortality was a simulation model based on hypothetical changes in smoking habits [Citation11]. This model was updated with respect to non-smokers’ lung cancer risks and the relative risks of lung cancer related to different smoking habits. This time also the female populations were included.
Materials and methods
The information presented for Poland was available covering a 40-year period: 1963–2000. The population data, by age, sex and year, and population forecast come from the Central Statistical Office and the lung cancer mortality data from the Department of Epidemiology of Institute of Oncology in Warsaw. The frequencies of smoking habits were smoothed based on the survey done by Central Statistical Office in 1996 and 2004 [Citation12,Citation13].
The information presented on Finland comes from the Finnish Cancer Registry (lung cancer [Citation14]) and the Finnish National Public Health Institute (smoking frequencies were smoothed from the annual surveys [Citation15]). The data relating to population numbers and population forecast in Finland are from Statistics Finland.
The Polish data concerning lung cancer are based on mortality rates, whereas data for Finland are based on incidence rates. This is due to incompleteness in the national Polish cancer registration [Citation16]. However, given the short survival time of the patients, the lung cancer mortality rate in Poland can be treated as a very close approximation to the incidence rate [Citation17,Citation18]. When standardizing the rates for age, the direct method with world standard population was used.
The present model was based on model-based smoothing of the smoking habit – specific risk ratios estimated for males and females in Europe by an international team of researchers [Citation19]. The models accounted for the age and sex as well as the length of smoking, number of cigarettes and time since stopping and were used to interpolate and extrapolate the relative risks compared to life-long non-smokers, as the categorization of smoking variables did not directly apply to the current study. The details of the models and the matrices of smoking habits are available on request from the first author.
The relative risk of lung cancer depends on the duration of smoking and the number of cigarettes smoked per day. The risk for both sexes rapidly increases with the increased number of cigarettes smoked and extended time period. For example, for males who smoke 40 cigarettes per day for 50 years the risk of lung cancer is 80 times higher than for non-smokers. For females the respective figure is 55. Similar relations are noticeable for ex-smokers. The level of relative risk decreases when the non-smoking period is increased and the number of cigarettes is reduced. Irrespective of these, the risk level does not reach that observed for non-smokers ().
Figure 1. Relative risk of lung cancer in male population in relation to duration and daily number of smoked cigarettes, model-based values on European study [Citation19].
![Figure 1. Relative risk of lung cancer in male population in relation to duration and daily number of smoked cigarettes, model-based values on European study [Citation19].](/cms/asset/f0342080-5d74-4a2c-9bbf-b256e739a838/ionc_a_488247_f0001_b.gif)
Figure 2. Relative risk of lung cancer in female population in relation to duration and daily number of smoked cigarettes, model-based values on European study [Citation19].
![Figure 2. Relative risk of lung cancer in female population in relation to duration and daily number of smoked cigarettes, model-based values on European study [Citation19].](/cms/asset/2f60c087-d581-44e0-b843-04b1fa14ab0a/ionc_a_488247_f0002_b.gif)
Figure 3. Relative risk of lung cancer for male ex-smokers in relation to duration, daily number of smoked cigarettes and time since stopping (2.5–17.5 years)-model-based values on European study [Citation19].
![Figure 3. Relative risk of lung cancer for male ex-smokers in relation to duration, daily number of smoked cigarettes and time since stopping (2.5–17.5 years)-model-based values on European study [Citation19].](/cms/asset/8bdf4c08-521f-4268-9957-464f63ac5668/ionc_a_488247_f0003_b.gif)
Figure 4. Relative risk of lung cancer for female ex-smokers in relation to duration, daily number of smoked cigarettes and time since stopping (2.5–17.5 years)-model-based values on European study [Citation19].
![Figure 4. Relative risk of lung cancer for female ex-smokers in relation to duration, daily number of smoked cigarettes and time since stopping (2.5–17.5 years)-model-based values on European study [Citation19].](/cms/asset/ccc9dd45-e2bb-47f0-aac0-5bb8bbab7489/ionc_a_488247_f0004_b.gif)
The non-smokers’ lung cancer rates were obtained from the smoothed results by Thun et al. [Citation20,Citation21]. For comparison, also the lung cancer rates for non-smokers from another source were utilized [Citation22]. The both models reproduced the observed lung cancer data in five-year age groups from 1996 reasonably well. The IARC models [Citation21] were applied to the Finnish and Polish male and female non-smokers up to 2030.
The operative principle of the simulation model used in forecasting presented is the following: Let us take a certain population stratified by sex and age in five-year groups with specified smoking habits and a population forecast. Populations are subject to changes in smoking habits (these are the variables that can be modified). The overall death risks related to smoking were used to determine the number of smokers who died as a result of smoking [Citation11]. Thus, a new population is established with new smoking habits for which it is possible to calculate the number of lung cancer cases.
Various future scenarios were defined by alternative hypothetical developments in smoking habits (). Two of the analyzed scenarios (SPes, SOpt) are quite extreme and determine limits of lung cancer incidence. The remaining scenarios (except for the scenario S0, ) represent more plausible fractions of persons quitting smoking in the future. In principle, also scenarios with different other percentages of quitters could have been tested. The basic scenario (S20) assumes that in every five-year period 30% of non-smokers aged 10–14, 15% of non-smokers aged 15–19 and 5% of those aged 20–24 years would start smoking and at the same time 20% of smokers in each smoking category would quit it. The number of cigarettes smoked by the beginners was assumed according to the observed dose distribution in the most recent year of the prediction and is not modified in particular scenarios ().
Table I. The different scenarios used in the predictions.
Results
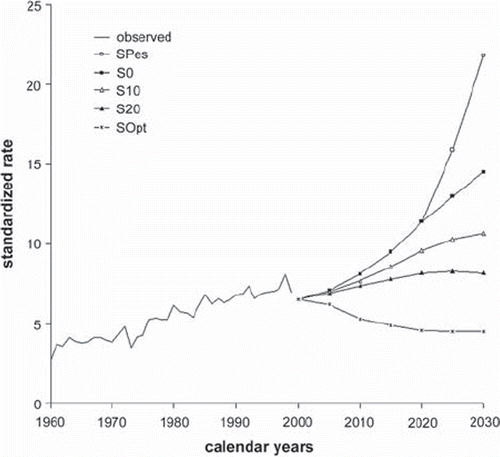
A scenario of continuation of the previous lung cancer mortality trend among men in Poland suggests that the mortality rate would go from value above 60/105 down to 40/105 in 2030 if the number of smokers was reduced by 20% in each five-year period (S20) (). If in subsequent five-year periods 10% of smokers quitted smoking the mortality rates would not decrease but remain on the same level (S10). In extreme, the most pessimistic scenario, if all young people aged 10–24 started smoking and nobody quitted, in 2030 the lung cancer mortality rate among the male population in Poland would increase to approximately 125/105 (SPes). If the pattern of smoking initiation was as in other scenarios and nobody quitted smoking, the favorable trend that is noticeable today would be reversed and the mortality rate would increase to 104/105 (S0). However, on the other hand, in the most optimistic scenario, if everybody quitted smoking immediately, the mortality rate would be decreased to 8/105 (SOpt), i.e. towards the level observed among non-smokers.
Figure 5. Prediction of lung cancer mortality among males in Poland: the rates are per 105 person-years and have been standardized for age to the World Standard Population. For the description of different scenarios see .
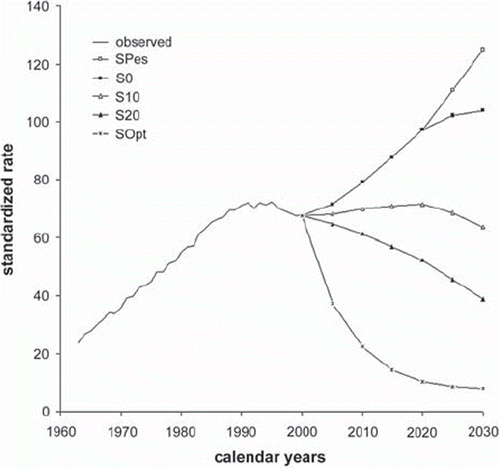
Among women the same scenarios are as follows (): in 2030, if in subsequent five-year periods 20% of smokers quit smoking, the mortality rate would still rise up to 15/105 (S20). If 10% quit smoking, the trend that is noticeable today would be continued and the mortality rate would still increase to 20/105 (S10). If all young women aged 10–24 started smoking and nobody quitted, the lung cancer mortality rate among the female population would increase to approximately 47/105 (SPes). The mortality rate would go down to 7/105 only if everybody quit smoking immediately (SOpt). In this case the mortality rate would be decreased close to the level observed among non-smokers.
Figure 6. Prediction of lung cancer mortality among females in Poland: the rates are per 105 person-years and have been standardized for age to the World Standard Population. For the description of different scenarios see .
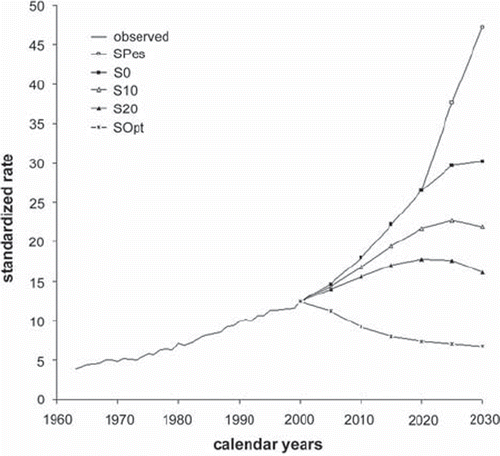
The ever-decreasing trend in lung cancer incidence rate among males in Finland, which started at the beginning of the 1980s, will continue provided that 20% of smokers in each smoking category quit this habit for good (). A 10% drop in the number of smokers will maintain the incidence rate on the current level. Assuming that nobody quits and everybody starts smoking, within 30 years the incidence rate will be almost the same as reported at the beginning of the 1960s.
Figure 7. Prediction of lung cancer incidence among males in Finland: the rates are per 105 person-years and have been standardized for age to the World Standard Population. For the description of different scenarios see .
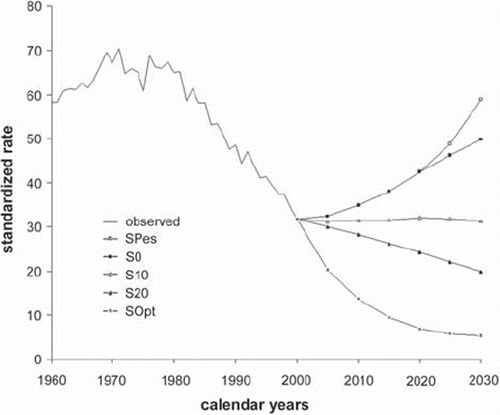
Among Finnish women, the rising trend in lung cancer incidence will continue provided that less than 20% of smokers in each smoking category quit the smoking habit (). A 20% drop in the number of smokers will maintain the incidence rate on the current observed level.
Discussion
Until the beginning of the 1990s the lung cancer mortality rate among males in Poland demonstrated a steady rising tendency. Since then, the trend leveled off, but a drop in the mortality rate can be expected only when in each five-year period 20% of male smokers give up the habit. Eliminating the habit for good (as demonstrated in the SOpt scenario) would yield a decrease in the lung cancer mortality rate close to the level reported for non-smokers, i.e. to five to six deaths per 100 000 people in a year. The same predictions hold true for Finland and Poland alike. This is because the same non-smoker rates were assumed for both countries.
A decrease in lung cancer rates for females in Finland and in Poland is possible only in the scenario which assumes an efficient elimination of the smoking habit. Otherwise the relevant rates would remain on the same level (i.e. in the scenario which assumes a 20% drop in the number of smokers in each smoking category) or would actually increase (in the remaining scenarios). Such results are attributed to the postponed consequences of smoking habits already adopted in the population.
The model developed by Hakulinen and Pukkala [Citation11] allows the observation of changes in lung cancer incidence rates on the basis of current and changed smoking habits in a population. The model also incorporates estimated relative risks for Europeans depending on the number of cigarettes smoked, duration of smoking and length of a non-smoking period. In the model, lung cancer incidence is calculated for every five-year generation in each consecutive five-year period of the prediction (for instance for women aged 40–44 years in 2000, next for the same birth cohort five years older, aged 45–49 years in 2005, and so on). Consequently, the model takes into account differences in tobacco smoke exposure between birth cohorts. This property has a special importance for the population of Polish women born during the war and shortly afterwards, which is characterized with very high percentage of smokers [Citation7,Citation8].
Just a few European countries have sufficiently detailed data on tobacco smoking distribution, according to the above characteristics. Additionally, the credibility and quality of these data on smoking habits has been questioned [Citation23]. The predictions of lung cancer incidence in European populations have mostly been based on mathematical models extrapolating the incidence rates [Citation1,Citation24,Citation25], not on simulations.
This model, despite its complexity and assumptions on smoking patterns in population, does not takes into account other lung cancer risk factors than tobacco. About 17% of lung cancer cases in men and 2% in women in Finland in 2000 might be eliminated by affecting occupational exposures, including exposure to asbestos [Citation26]. Only 1% of lung cancer cases in males in Finland in 2000 could be eliminated by affecting the exposure to radon [Citation27]. The corresponding fraction in females was estimated to be 4%. Due to the multiplicative interactions between causal factors, most of the cases that could be eliminated affecting another exposure could also be eliminated by giving up smoking, particularly in males. Given these estimates, one can assume that the trends of changes in lung cancer incidence will be to a large extent in accordance with the model results. This has also been observed with the predictions made earlier by using the model [Citation28].
The results obtained clearly indicate that cutting down on the number of smokers translates directly into a considerable reduction of the lung cancer incidence rate. Lung cancer is a disease to be easily avoided. Smoking cessation is the best way to reduce risk of lung cancer among human population. There are many epidemiological studies (both cohort studies and case-control) that confirm this [Citation6]. The predictions clearly indicate that there are tremendous opportunities to reduce the lung cancer incidence rate in both Finland and Poland, provided that smoking habits can be changed. Even though the paper focuses on lung cancer incidence, the benefits coming from reducing smoking in population where smoking is common, would contribute to decline of morbidity and mortality from all tobacco-related diseases, such as cardiovascular diseases, chronic obstructive pulmonary disease and other tobacco-related cancers [Citation29].
The current model assumes a uniform starting and stopping behavior by population subgroups, particularly social classes. It is possible that the upper, more educated classes are more likely to stop and less likely to start. This is clearly a limitation that can, without introduction of the social class into the model, only be addressed indirectly by the model used. It can be done by assuming smaller frequencies of stopping for heavier smokers that are likely to be represented more in the lower social classes. This is one important way to extend the current study. Explicit inclusion of social class in the model is, however, not likely to be possible due to lack of prediction of social class-specific populations into the future.
These predictions were developed thanks to a special interactive model which was designed, and the exercise can be repeated for any population provided that there is enough information about smoking habits available. The software was developed on the basis of program originally created by Hakulinen and Pukkala for the Finnish male population. A primary version of this software was generated in Algol language, whereas the present version has been written in Pascal for the DOS system. There is no up-grade for Windows system. The software is available from the two first authors.
Populations continue to puff their way to smoky, cancerous death. As Roberto Benigni [Citation30] would say – “Life is Beautiful, so why waste it if there is a definite link between smoking and lung cancer”.
Acknowledgements
The work was supported by the MaDaMe Programme of the Academy of Finland. There is no conflict of interest to be declared.
References
- Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581–92.
- Zatoński W, Didkowska J. Closing the gap: Cancer in Central and Eastern Europe (CEE). Eur J Cancer 2008;44:1425–37.
- Tyczynski JE, Bray F, Parkin DM. Lung cancer in Europe in 2000: Epidemiology, prevention, and early detection. Lancet Oncol 2003;4:45–55.
- Doll R, Peto R. The causes of cancer. Quantitative estimates of avoidable risk of cancer in the United States today. Oxford: Oxford University Press; 1981.
- Doll R. Fifty years of research on tobacco J Epid Biostat 2000;5:321–9.
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519.
- Zatoński W, Przewozniak K. Palenie tytoniu w Polsce: Postawy, następstwa zdrowotne i profilaktyka. (Tobacco smoking in Poland: attitudes, health consequences and prevention). Warszawa: Centrum Onkologii-Instytut im. Marii Sklodowskiej-Curie; 1999.
- Ramlau R, Didkowska J, Wojciechowska U, Tarkowski W. Palenie tytoniu w Wielkopolsce w końcu XX wieku (Tobacco smoking in Wielkopolska towards the end of 20th century). Pneumonol Alergol Pol 2005;73:128–34.
- Heloma A, Nurminen M, Reijula K, Rantanen J. Smoking prevalence, smoking-related lung diseases, and national tobacco control legislation. Chest 2004;126:1825–31.
- Statistics Finland. Tobacco statistics 2007. Official statistics of Finland, Health 2008. Helsinki: Statistics Finland; 2008.
- Hakulinen T, Pukkala E. Future incidence of lung cancer: Forecasts based on hypothetical changes in the smoking habits of males. Int J Epidemiol 1981;10:233–40.
- Stan zdrowia ludnosci Polski w 1996 roku. Raport przygotowany na podstawie ankietowego badania stanu zdrowia ludności przeprowadzonego w 1996 roku. (Health population status in Poland in 1996. Report prepared on the basis of Health Interview survey carried out in 1996). Warszawa: GUS (Central Statistical Office); 1997.
- Stan zdrowia ludnosci Polski w 2004 roku. (Health population status in Poland in 2004). Warszawa: GUS (Central Statistical Office); 2006.
- Finnish Cancer Registry. Cancer in Finland 2006 and 2007. Helsinki: Cancer Society of Finland Publ. No 76; 2009.
- Helakorpi S, Patja K, Prättälä R, Uutela A. Health behaviour and health among the Finnish adult population, Spring 2007. Helsinki: Publications of the National Public Health Institute B6; 2008.
- Didkowska J, Wojciechowska U, Tarkowski W, Zatoński W. Nowotwory złośliwe w Polsce w 2000 roku (Cancer in Poland in 2000). Warszawa: Centrum Onkologii – Instytut im. M. Skłodowskiej-Curie (The Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology); 2003.
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R, EUROCARE Working Group. EUROCARE-4. Survival of patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91.
- Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, . EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995–99: Results of the EUROCARE-4 study. Lancet Oncol 2007;8:773–83.
- Simonato L, Agudo A, Ahrens W, Benhamou E, Benhamou S, Boffetta P, . Lung cancer and cigarette smoking in Europe: An update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer 2001;91:876–87.
- Thun MJ, Myers DG, Day-Lally C, Namboodiri MM, Calle EE, Flanders WD, . Age and exposure-response relationships between cigarette smoking and premature death in cancer prevention study II. Changes in cigarettes related disease risks and their implication for prevention and control. Smoking and Tobacco Control Monograph No. 8, NIH Publication No. 97-4213. Bethesda MD: US Department of Health and Human Services, National Institutes of Health; 1997.
- International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Vol 83. Lyon: IARC; 2004.
- Boffetta P, Järvholm B, Brennan P, Nyrén O. Incidence of lung cancer in a large cohort of non-smoking men from Sweden. Int J Cancer 2001;94:591–3.
- West R, Zatonski W, Przewozniak K, Jarvis MJ. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev 2007;16:820–2.
- Dyba T, Hakulinen T. Do cancer predictions work? Eur J Cancer 2008;44:448–53.
- Brennan P, Bray I. Recent trends and future directions for lung cancer mortality in Europe. Br J Cancer 2002;87:43–8.
- Dreyer L, Andersen A, Pukkala E. . OccupationOlsen J, Andersen A, Dreyer L, Pukkala E, Tryggvadottir L, Gerhardsson de Verdier M, . Avoidable cancers in the Nordic countries. APMIS 1997;105(Suppl 76):68–79.
- Winther JF, Ulbak K, Dreyer L, Pukkala E, Østerlind A. . RadiationOlsen J, Andersen A, Dreyer L, Pukkala E, Tryggvadottir L, Gerhardsson de Verdier M, . Avoidable cancers in the Nordic countries. APMIS 1997;105(Suppl 76):83–99.
- Bray F, Møller B. Predicting the future burden of cancer. Nat Rev Cancer 2006;6:63–74.
- Peto R, Lopez AD, Boreham J, Thun M, Heath C Jr. Mortality from smoking in developed countries 1950–2000. Oxford: Oxford University Press; 1994.
- Ferri E, Braschi G, Begnini R. Life is beautiful [Motion picture]. Italy: Miramax. 1998.