To the Editor,
Erlotinib, an orally administered epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), has shown promising antitumor activity for non-small-cell lung cancer (NSCLC) as compared with the best supportive care [Citation1]. It inhibits the intracellular phosphorylation of the tyrosine kinase domain associated with EGFR in cancer cells. This activity also occurs in tissues that normally express EGFR, such as the basal and suprabasal layers of the epidermis, sebaceous glands, and the outer root sheath of hair follicles [Citation2], which results in papulopustules, pruitus, paronychia, xerosis, and telangiectasias. Recently, several researchers have independently reported cases with hair alterations as rarely occurring adverse events that were potentially induced by erlotinib treatment. However, it has never been investigated how rare these events are. In this report, we present our case series in a single institution that developed toxicity of cosmetic hair alterations during erlotinib treatment, and then focus on the frequency of these events.
An 81-year-old woman, diagnosed with advanced pulmonary adenocarcinoma in December 2005 and treated with platinum-based chemotherapy, was admitted to our hospital in July 2008 due to progression of her disease. We treated her with erlotinib at a dose of 150 mg once daily, which achieved a partial response. The deletion of exon 19 in the EGFR gene was detected retrospectively using a specimen previously obtained by transbronchial biopsy.
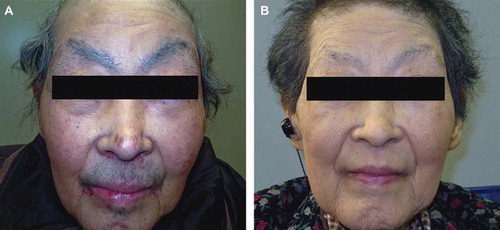
At first, toxicity was mild and manageable with only a grade 2 cutaneous rash. However, around five months later (December 2008) she developed hair alterations primarily on her face. Gradually, her eye brows, eye lashes, and beard became darker, thicker, and elongated (). Hormonal examinations revealed no abnormal serum levels of testosterone or estrogen, and no other agents had been administered that could have induced the adverse event. We continued her erlotinib treatment without any supportive care because the event did not appear life-threatening. In April 2009, erlotinib was discontinued due to the progression in treatment of her disease, and we observed that her hair alterations were resolved three months after discontinuing this drug ().
Figure 1. Photography images of the face showing hair alterations (hypertrichosis) induced by erlotinib. Her eye brows, eye lashes, and beard became darker, thicker, and elongated (A). However, these adverse events were resolved after discontinuing erlotinib because of disease progression (B).
Since the approval of erlotinib in Japan in January 2008, 33 patients with refractory NSCLC have received erlotinib monotherapy in our hospital. A total seven of 12 female patients developed hair alterations during their treatment, and surprisingly these adverse events were not observed in any male patient. shows their clinicopathological features, including the case presented above. All seven female patients had been diagnosed with adenocarcinoma and mutations of the EGFR gene were detected in six assessable patients. The time to first presentation of hair alterations ranged from 72 to 270 days. For five patients, these toxicities resolved several months after discontinuation of erlotinib treatment due to progressed disease. An objective response was observed for six patients and one had stable disease. Response durations in these patients were over four months and two patients are still receiving erlotinib treatment.
Table I. Clinicopathological features of patients with NSCLC showing hair alterations with erlotinib treatment.
In published series, several researchers have independently reported a total of 20 cases that presented with hair alterations possibly induced by the EGFR inhibitors, erlotinib, gefitinib, and cetuximab [Citation3–6]. According to these reports, hair alterations appeared to occur principally on a patient's face and were characterized as hypertrichosis of the eyelashes, brows, and beards or eyelash trichomegaly, accompanied by trichorrhexis, which continued for several months after discontinuation of the treatment. All of our cases also demonstrated similar manifestations and clinical courses. The majority of previously reported cases manifested eyelash trichomegaly, whereas hypertrichosis of the brows and beards occurred only in a small proportion of cases. Each researcher emphasized that the adverse event was quite rare when presenting their cases, while our results clearly showed a relatively high proportion of patients with hair alterations.
Androgenic sex hormones that bind with globulin such as testosterone can cause hypertrichosis [Citation7]. Additionally, certain drugs, such as interferon alpha, cyclosporine, and tacrolims, have been reported to lead to the development of trichomegaly [Citation3]. Our patients did not have elevated hormonal serum levels nor took drugs that could cause hair growth, and most of them had relief from toxicity with discontinuation of erlotinib therapy. Thus, our case series suggests that hair alterations might develop by the direct effect of EGFR inhibitors on hair follicles.
The possibility of developing hair alterations due to EGFR inhibitors was discussed in the previous reports. EGFR plays an important role for regulating the transformation from an anagen (or growth phase) to a catagen (or regressive phase) [Citation8]. Inhibiting the EGFR activity of hair follicles might alter their growth cycle and halt the anagen-catagen transformation, resulting in hair alterations [Citation6]. Future investigations are warranted in order to identify the relevant mechanisms.
We found a relatively high incidence of these events in female patients as observed in previous literature; 15 (75%) of 20 cases were female [Citation3–7]. Simply put, hair alterations can be easily detected in females. In addition, our results also appear interesting in view of the correlation between hair alterations and the treatment efficacy, as the subpopulation with female gender, adenocarcinoma histology, EGFR mutations, and smoking status (non-smoking) were potentially responsive to EGFR-TKIs [Citation1,Citation9]. Indeed, the majority of our cases responded to erlotinib and achieved favorable progression-free survival times. It would be intriguing to confirm these correlations in further studies.
In conclusion, we found a relatively high proportion of patients with hair alterations during erlotinib treatment. Although this is not life-threatening, it should be noticed with careful observations cosmetically, especially for female patients. We believe that an evaluation using a large-scale epidemiologic study is required in order to elucidate the association between erlotinib and these events.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, . National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005; 353:123–32.
- Fox LP. Pathology and management of dermatologic toxicities associated with anti-EGFR therapy. Oncology 2006; 20:26–34.
- Braiteh F, Kurzrock R, Johnson FM. Trichomegaly of the eyelashes after lung cancer treatment with the epidermal growth factor receptor inhibitor erlotinib. J Clin Oncol 2008;26:3460–2.
- Alexandrescu DT, Kauffman CL, Dasanu CA. Persistent hair growth during treatment with the EGFR inhibitor erlotinib. Dermatol Online J 2009;15:4.
- Márquez G, Herrera-Acosta E, Vidal I, Galvany L, Iglesias M, Umbert P. A case of trichomegaly of the eyelashes and facial hypertrichosis induced by erlotinib (Tarceva). Int J Dermatol 2009;48:97–8.
- Montagut C, Grau JJ, Grimalt R, Codony J, Ferrando J, Albanell J. Abnormal hair growth in a patient with head and neck cancer treated with the anti-epidermal growth factor receptor monoclonal antibody cetuximab. J Clin Oncol 2005;23:5273–5.
- Gryngarten M, Bedecarràs P, Ayuso S, Bergadà C, Campo S, Escobar ME. Clinical assessment and serum hormonal profile in prepubertal hypertrichosis. Horm Res 2000;54:20–5.
- Hansen LA, Alexander N, Hogan ME, Sundberg JP, Dlugosz A, Threadgill DW, . Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am J Pathol 1997;150:1959–75.
- Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, . Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: Results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527–37.