To the Editor,
Extranodal NK/T cell lymphoma (ENKTL) is a distinct entity in the World Health Organization (WHO) classification that is almost always associated with Epstein-Barr virus (EBV) infection and is more common among Asians. Although local radiotherapy is fairly effective in controlling early stage ENKTL, prognosis is dismal for patients with advanced stage ENKTL [Citation1]. Currently, there is no standard first line therapy for the treatment of advanced ENKTL. Anthracycline-based regimens such as CHOP (cyclophosphamide, doxorubicin, vinciristine and prednisolone) yield dismal response rates (<15%), partly because the neoplastic NK cells over-express p-glycoprotein, a mediator of the multidrug resistance (MDR) phenotype [Citation2]. Other novel regimens incorporating L-asparaginase have reported better results [Citation3] but await further confirmation. We present a 30-year-old lady with advanced ENKTL who achieved complete response with a novel treatment regimen after progressing through L-asparaginase based chemotherapy.
Our patient presented with a five month history of fever, left eye redness and neck swelling. Computed tomography (CT) revealed generalized lymphadenopathy involving cervical, axillary, paratracheal, para-aortic, iliac and inguinal nodes as well as massive hepatosplenomegaly with splenic infarcts. Magnetic resonance imaging (MRI) brain/orbits showed no intracranial disease. The EBV DNA titre at diagnosis was 11 297 copies/ml. Biopsies taken from the nasopharyngeal soft tissue mass and the left submandibular lymph node revealed a neoplastic lymphoid population that expressed the pan-T cell markers CD2 and CD3, the NK cell marker CD56 and the cytotoxic molecule TIA1, with in situ hybridization for EBV encoded RNA (EBER) strongly positive. Bone marrow trephine biopsy revealed similar histological findings.
The patient thus had Stage IV nasal ENKTL, with an international prognostic index (IPI) score of 3 (elevated lactate dehydrogenase, advanced Ann Arbor Stage and multiple extranodal disease sites). She was started on an L-aspariginase based regimen that comprised dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide (SMILE), a combination that had previously shown promising results in Phase I studies for patients with advanced or refractory ENKTL [Citation2]. The chemotherapy was given over 14 days with growth factor support, and repeated every 28 days. Disease response was evaluated clinically, radiologically with positron emission tomography (PET)-CT, and with serial EBV DNA titres. Despite initial response, there was disease progression following cycle 3 of SMILE, with rising EBV DNA titres. Her treatment was also complicated by methotrexate retention and prolonged neutropenia.
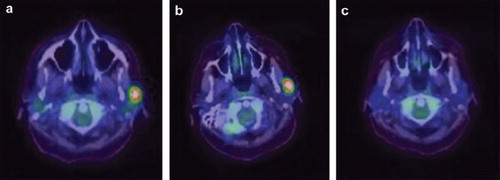
Treatment was then switched to a novel regimen comprising bortezomib, gemcitabine, ifosfamide and oxalipaltin (B-GIFOX) given over four days with growth factor support, repeated every two weeks. PET CT after two cycles revealed near complete response, with EBV titres rendered undetectable for two consecutive readings (see ). The chemotherapy was well tolerated with no significant hematologic or extra-hematologic toxicities. The patient received a total of four cycles of chemotherapy with a progression free survival of two months.
Discussion
This patient progressed despite receiving a fairly intensive L-asparaginase based therapy and responded to B-GIFOX with minimal toxicity. An effective salvage therapy should ideally have significant cytoreductive activity, minimal extra-hematologic toxicity and be conveniently administered.
Our regimen, B-GIFOX, was developed based on a combination of known efficacy and toxicity data of cytotoxic agents in ENKTL, and results from our own pre-clinical studies using these agents in our laboratory [Citation4,Citation5]. Gemcitabine is a deoxycytidine analogue that has demonstrated single agent efficacy in relapsed or refractory aggressive lymphomas [Citation6,Citation7]. Additionally, it compares favorably to its parent compound, cytarabine, in achieving highly effective intracellular concentrations [Citation8]. Ifosfamide is a bifunctional alkylator that is a highly active non-cross resistant drug in second line therapy of lymphoma [Citation9]. Both gemcitabine and ifosfamide are unaffected by the MDR phenotype. Oxaliplatin is a platinum derived agent that has shown similar efficacy to cisplatin as a key agent in second line treatment of lymphoma [Citation10]. Importantly, oxaliplatin has a more favorable extra-hematologic toxicity profile than cisplatin, thus facilitating the delivery of gemcitabine and ifosfamide at adequate dose intensity. In addition, strong synergy has been documented between gemcitabine and oxaliplatin in several clinical studies [Citation11]. Bortezomib is a proteasome inhibitor that has displayed remarkable single agent anti-tumor activity in a variety of hematologic malignancies. We tested these drugs as single agents in an EBV positive, NK lymphoma xenograft model in SCID mice with reported encouraging results. In these studies, ifosfamide and bortezomib were effective in inducing sustained tumor regression, while gemcitabine and oxaliplatin also caused tumor regression [Citation5].
We believe that B-GIFOX is a promising regimen in the treatment of ENKTL and are currently designing a prospective phase II study to validate its efficacy.
NOTICE OF CORRECTION
The Early Online version of this article published online ahead of print on 12 Jan 2011 contained an error on page 1. Several of the contributing authors was left out. This has been corrected for the current version.
References
- ST Lim, Siew WH, Quek R, LC Lim, SP Yap, Loong EL, . Comparative analysis of extranodal NK/T cell lymphoma and peripheral T cell lymphoma: Significant differences in clinical characteristics and prognosis. Eur J Haematol 2008;80:55–60.
- Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P-glycoprotein expression on normal and abnormally natural killer cells and inhibition of P-glycoprotein function by cyclosporine A and its analogue, PSC833. Blood 1999;93: 599–606.
- Yamaguchi M, Suzuki R, Kwong YL, Kim WS, Hasegawa Y, Izutsu K, . Phase I study of dexamethasone, methotrexate, ifosfamide, L-Asparaginase and etoposide (SMILE) chemotherapy for advanced stage, relapsed or refractory extranodal natural killer (NK)/T cell lymphoma and leukemia. Cancer Sci 2008;99:1016–20.
- Loong SL, Hwang JS, Lim ST, Yap SP, Tao M, Chong TW, . An Epstein Barr Virus positive natural killer lymphoma xenograft derived for drug testing. Leuk Lymphoma 2008; 49:1161–7.
- GC Koo, W Esa, ST Lim, SY Tan, S Loong. Evaluation of the efficacy of selected chemotherapeutic and immunotherapeutic drugs in NK/T non-Hodgkin's lymphoma xenograft model. Poster 023. BIT Life Sciences’ 2nd Annual World Cancer Congress 2009, Beijing, China.
- Fosså A, Santoro A, Hiddemann W, Truemper L, Niederle N, Buksmaui S, . Gemcitabine as a single agent in the treatment of relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol 1999;17:3786–92.
- Savage DG, Rule SA, Tighe M, Garrett TJ, Oster MW, Lee RT, . Gemcitabine for relapsed or resistant lymphoma. Ann Oncol 2000;11:595–7.
- Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic and phase I studies of Gemcitabine. Semin Oncol 1997;24(2 Suppl 7):S7-2–S7-7.
- Hagemeister FB, Diehl V. The role of ifosfamide in cytoreduction, stem cell mobilization and high dose therapy in relapsed/refractory malignant lymphomas. Eur J Haematol Suppl 2001;64:1–2.
- Oki Y, McLaughlin P, Pro B, Hagemeister FB, Bleyer A, Loyer E, . Phase II study of oxaliplatin in patients with recurrent or refractory non Hodgkin's lymphoma. Cancer 2005;104:781–7.
- Faivre S, Raymond E, Woynarowski JM, Cvitkovic E. Supraadditive effect of 2′,2′-difluorodeoxycytidine (gemcitabine) in combination with oxaliplatin in human cancer cell lines. Cancer Chemother Pharmacol 1999;44:117–23.