To the Editor,
Gastrointestinal stromal tumours (GISTs) may occur in the entire length of the gastrointestinal tract accounting for 1–3% of all gastrointestinal malignancies [Citation1]. Surgery is first-line treatment for patients with primary resectable GIST [Citation2,Citation3]. Approximately half of the patients will relapse within five years despite complete surgical resection [Citation4], and standard chemotherapy and radiotherapy is not effective [Citation5]. With an understanding of the key role of the KIT tyrosine kinase expression [Citation6] and the introduction of the tyrosine kinase inhibitor (TKI) imatinib, a new era in the management of this tumour entity began about ten years ago. Imatinib shows a pronounced effect in metastatic GIST, albeit often temporarily [Citation7,Citation8]. There is also a growing interest for neoadjuvant treatment with TKIs in patients with large or marginally resectable GISTs [Citation8,Citation9].
Computed Tomography (CT) has conventionally been used for monitoring patients with GIST. However, new targeted therapies have revealed the insufficiency of the solid tumour size-based response assessment criteria, since initial treatment effect seldom is observed as tumour volume reduction [Citation10]. Decreased tumour size and reduced attenuation on CT imaging have been used to document GIST response to imatinib treatment [Citation10].
During the last few years, positron emission tomography/computed tomography (PET/CT) has become an increasingly important tool for response evaluation in patients with cancer. Most GISTs show high uptake of 18F-fluorodeoxyglucose (18F FDG) when imaged by PET, and treatment response can be observed early [Citation11], even as early as 24 hours after onset of treatment [Citation12]. However, some GISTs do not show 18F FDG uptake, still being overtly malignant [Citation13].
Recently, diffusion-weighted magnetic resonance imaging (DW MRI) has been applied to depict abdominal and pelvic malignancies [Citation14], but there is only one case report where DW MRI has been used for the diagnosis of GIST [Citation15]. Malignant tumours usually show high intensity on DW MRI because of their high cellular density limiting the motion of water molecules [Citation15]. During TKI treatment the cellular density is anticipated to decrease and thereby also the diffusion signal [Citation16].
To our knowledge, DW MRI has not been used for the assessment of treatment response of GISTs. This report presents a case of rectal GIST given neoadjuvant imatinib treatment, where DW MRI, along with other established response evaluation imaging modalities (CT, PET and dynamic contrast enhanced (DCE) MRI) were used to assess treatment response.
Case report
A 59-year-old male consulted his general practitioner because of constipation and two episodes of rectal bleeding. CT revealed a 12.5 cm tumour in the dorsal rectum wall in close proximity to the mesorectal fascia (). The attenuation coefficient in the tumour was 65 HU. Conventional MRI demonstrated a 470 ml, highly vascularised tumour ( and ). The tumour demonstrated high signal intensity at DW MRI (). Apparent diffusion coefficient (ADC) was approximately 1.0 × 10−3 mm2/s, typical value for solid tumours (). 18F FDG PET/CT prior to imatinib administration showed high 18FDG tumour-uptake and a Standardized Uptake Value (SUV) of 14.7 (). Histological evaluation of a needle biopsy confirmed the diagnosis of a CD117+ GIST with a mitotic count of 14 in 50 high-power fields (HPF) and mutations in exon 11 (Kit: c.1667_1672het_delAGTGGA, p. Trp557_Lys558del).
Figure 1. Morphological and functional images acquired prior to treatment (upper row) and after onset of neoadjuvant tyrosine kinase inhibitor treatment (bottom row) in a 59-year-old GIST patient. Contrast-enhanced CT (a and g), T2-weighted MRI (b and h), contrast-enhanced MRI (c and i), DW MRI (d and j), fusion of apparent diffusion coefficient (ADC) maps (obtained from DW MRI) and T2 weighted MRI (e and k) and 18F-FDG PET (f and l).
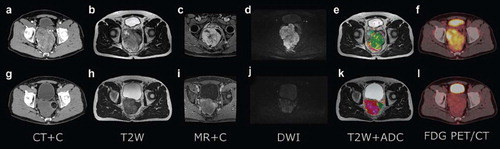
To facilitate resectability, the patient was given neoadjuvant treatment with imatinib (Glivec® 400 mg daily). 18F FDG PET/CT after two weeks of imatinib treatment showed metabolic activity () with a SUV of 3.5 (76%). Two months after onset of imatinib treatment CT showed a decrease in attenuation to 35 HU (46%) (). MRI performed prior to surgery, following six months of imatinib treatment, showed reduced tumour volume to 174 ml (63%) () and pronounced decrease in vascularisation (). Signal intensity at DW MRI was dramatically reduced (). ADC was approximately 2.0 × 10−3 mm2/s, typical value for non-cystic necrotic tissue (). A repeated CT performed approximately at the same time as MRI showed unchanged size and attenuation of the tumour as compared to the examination two months after onset of treatment.
Surgery was performed after seven months of imatinib treatment. There were no metastases in the abdominal cavity or in the liver. The postoperative course was uneventful, and the patient was discharged one week later. Imatinib was planned to continue for one year.
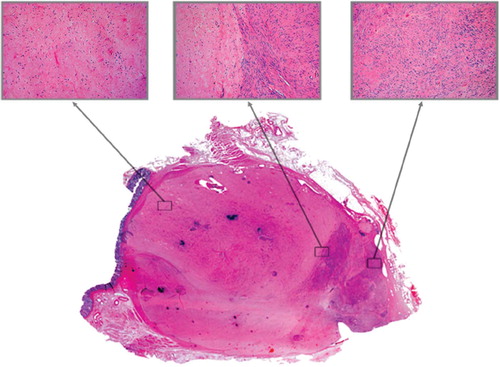
At histopathological examination the resected tumour was measured to 8 cm × 7.5 cm × 6 cm which is comparable to MRI-assessed volume. The histological and histochemical findings of the resected tumour confirmed the pre-treatment biopsy finding of a CD117+ GIST. Tumour consisted of large areas of necrotic tissue and scattered spindle cells () reflecting good treatment response, albeit heterogeneous. None of the imaging modalities reflected these variations or indicated the presence of residual tumour. More advanced imaging techniques are needed for this purpose.
Conclusion
This case report illustrates the potential of DW MRI for being a tool for monitoring of treatment response in GISTs following TKI therapy. Compared to the other established imaging modalities (PET, CT and DCE MRI), DW MRI do not rely on the use of ionising radiation, there is no need for administration of endogenous contrast agent or radioactivity, and the examination can be carried out in few minutes. The findings in this case report must, however, be confirmed in prospective studies with special emphasis on the establishment of optimal time-point for imaging and treatment response criteria.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): Definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol 2003;54:3–24.
- Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: Emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol 2004;11:465–75.
- Heinrich MC, Corless CL. Gastric GI stromal tumors (GISTs): The role of surgery in the era of targeted therapy. J Surg Oncol 2005;90:195–207.
- Samiian L, Weaver M, Velanovich V. Evaluation of gastrointestinal stromal tumors for recurrence rates and patterns of long-term follow-up. Am Surg 2004;70:187–91.
- DeMatteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: Before and after STI-571. Hum Pathol 2002;33:466–77.
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, . Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279(5350): 577–80.
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von MM, Benjamin RS, . Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626–32.
- Loughrey MB, Mitchell C, Mann GB, Michael M, Waring PM. Gastrointestinal stromal tumour treated with neoadjuvant imatinib. J Clin Pathol 2005;58:779–81.
- Katz D, Segal A, Alberton Y, Jurim O, Reissman P, Catane R, . Neoadjuvant imatinib for unresectable gastrointestinal stromal tumor. Anticancer Drugs 2004;15:599–602.
- Choi H. Response evaluation of gastrointestinal stromal tumors. Oncologist 2008;13(Suppl 2):4–7.
- Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, . Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 2001;344:1052–6.
- Shinto A, Nair N, Dutt A, Baghel NS. Early response assessment in gastrointestinal stromal tumors with FDG PET scan 24 hours after a single dose of imatinib. Clin Nucl Med 2008;33:486–7.
- Choi H, Charnsangavej C, de Castro FS, Tamm EP, Benjamin RS, Johnson MM, . CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: A quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619–28.
- Koh DM, Padhani AR. Diffusion-weighted MRI: A new functional clinical technique for tumour imaging. Br J Radiol 2006;79:633–5.
- Matsui T, Mitsui H, Sekigawa K, Kobayashi K, Okubo M, So E, . A case of a duodenal gastrointestinal stromal tumor diagnosed with the aid of diffusion-weighted magnetic resonance imaging. Clin J Gastroenterol 2009;2:384–7.
- Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging 2006;6:135–43.